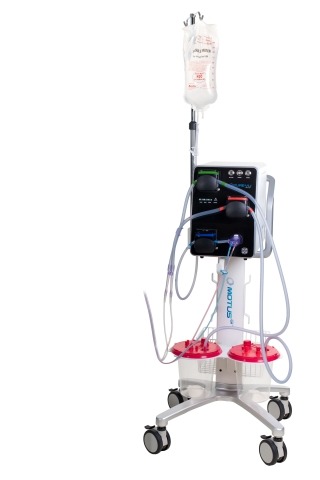
“We continue to strengthen our extensive global IP portfolio covering all aspects of the Pure-Vu System. This patent is particularly important as we look to the future expansion into the European market where approximately 5.6 million colonoscopy procedures were performed in 20181,” commented Tim Moran, Chief Executive Officer of Motus GI. “Additionally, establishing a robust IP portfolio is directly relevant to our plans to establish strategic partnerships to support global commercialization outside the U.S.”
The Pure-Vu System is a 510(k) U.S. Food and Drug Administration cleared medical device indicated to help facilitate the cleaning of a poorly prepared colon during the colonoscopy procedure. The device integrates with standard and slim colonoscopes to enable safe and rapid cleansing during the procedure while preserving established procedural workflow and techniques. The first generation of the Pure-Vu System has received CE mark approval in Europe and Motus GI intends to seek CE Mark approval for the Pure-Vu GEN2 System.
Source: Company Press Release






