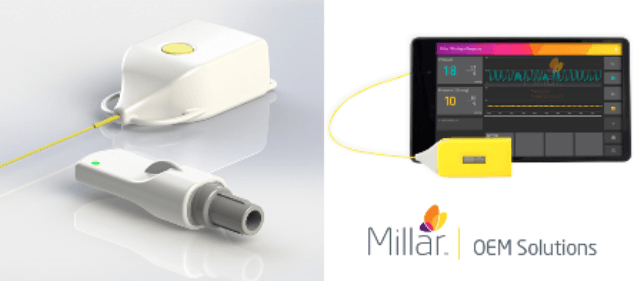
Millar announced the availability of a wireless pressure platform for integration in medical device technology.
The company said that the wireless platform allows OEM partnership opportunities for new device development in cardiovascular, respiratory, orthopedic, neurology and urodynamics applications.
Millar’s platform is designed to integrate with existing medical technology to enhance the quality of patient care by removing extraneous wires.
Millar innovation director David Budgett said: “When the signal is crucial, making it reliable using a wireless connection is the right thing to do.”
The wireless systems can connect with existing monitoring systems via Bluetooth data transfer, through the paired dongle, to maintain a wire-free environment for the patient.
Millar claims that its R&D team has experience in developing wireless devices and will use new technology from the company’s relationship with the University of Auckland.
Design of the current platform is claimed to be flexible and it can be customized to meet various product development specifications.
Real-time feedback is critical for trauma patients for continuous intra-compartmental pressures and the application of this technology can be crucial in such patients, who are unable to provide symptomatic feedback for discomfort in their extremities.
Catheter inserted with wireless, continuous pressure read-outs can reduce the need for multiple pressure measurements and can offer an immediate feedback to the care team if pressure begins to increase. Such preventative measurements can avoid loss of limbs from undiagnosed compartment syndrome.
Millar claims to possess nearly 50 years of microelectromechanical systems (MEMS) pressure sensor integration design and manufacturing, with compliance to blood pressure measurement standard AAMI BP-22 and IEC 60601, can provide potential partners with technology competence in invasive pressure monitoring for innovative device development.
The company recently stated that it will finalize its exit from the life sciences telemetry market on 31 December, this year.
Millar divested its telemetry product line to Kaha Sciences last year, but maintained a distribution partnership for the European markets.
To fully complete the transition, Millar will exit from its role of distributor sales and will maintain a business relationship with Kaha Sciences as an OEM pressure sensor integration solutions partner.




