Following the receipt of CE-mark, Microsure has made MUSA clinically and commercially available for use.
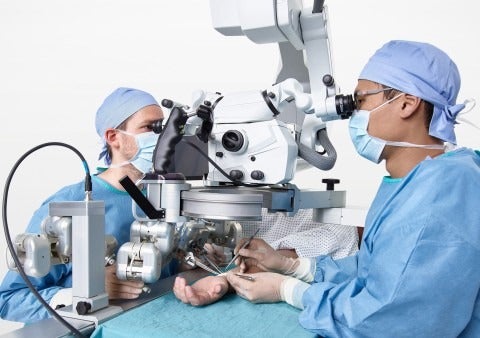
Developed by a team of microsurgeons and engineers precisely for microsurgical applications, MUSA is set to be the first surgical robot for open microsurgery.
MUSA is expected to provide enhanced precision for microsurgeons, and enables new interventions that are currently impossible to perform by hand.
The device was developed as a high-precision robotic assistant compatible with current operating techniques, workflow, instruments and other OR-equipment, to enhance the surgical performance by stabilizing and scaling down the surgeon’s movements during complex microsurgical procedures on sub-millimetre scale.
Microsure said that plastic surgeons at Maastricht University Medical Center+ were the first to use MUSA to surgically treat lymphedema in patients, and the device is capable of performing lymphatic surgery on lymph vessels smaller than 0.3mm in diameter.
In 2017, the super-microsurgical intervention with robot hands was initiated and the Maastricht University Medical Center+ is currently conducting various follow up clinical research projects with MUSA, in different fields of microsurgery.
The medical device company said that its MUSA would maximally benefit procedures that are complex surgeries on small tissue structures, when human precision is the limiting factor due to physiological tremor, poor accessibility or fatigue.
The procedures include Lymphovenous Anastomosis (LVA) surgery, pediatric vascalar surgery, free flap surgery, finger and hand replantation. Various studies and pilots are conducted to demonstrate the safety and effectiveness of the device’s clinical use.
Microsure said that its MUSA has also received the ISO 13485 certificate which marks another major milestone and the certification assures that the company complies with standards in quality management and regulatory compliance procedures to develop, manufacture, and test its products and services.
Founded by Eindhoven University of Technology and Maastricht University Medical Center in 2016, Microsure focuses on improving patients’ quality of life through developing robot systems for microsurgery.



