Medtronic has secured the US Food and Drug Administration (FDA) approval for its Evolut FX+ transcatheter aortic valve replacement (TAVR) system to treat severe aortic stenosis.
The Evolut FX+ TAVR system is indicated for symptomatic severe aortic stenosis patients across all risk categories, extreme, high, intermediate, and low, in the US.
Severe aortic stenosis is a condition where the aortic valve leaflets become stiff, thickened, and difficult to open and close, making the heart work harder to pump blood.
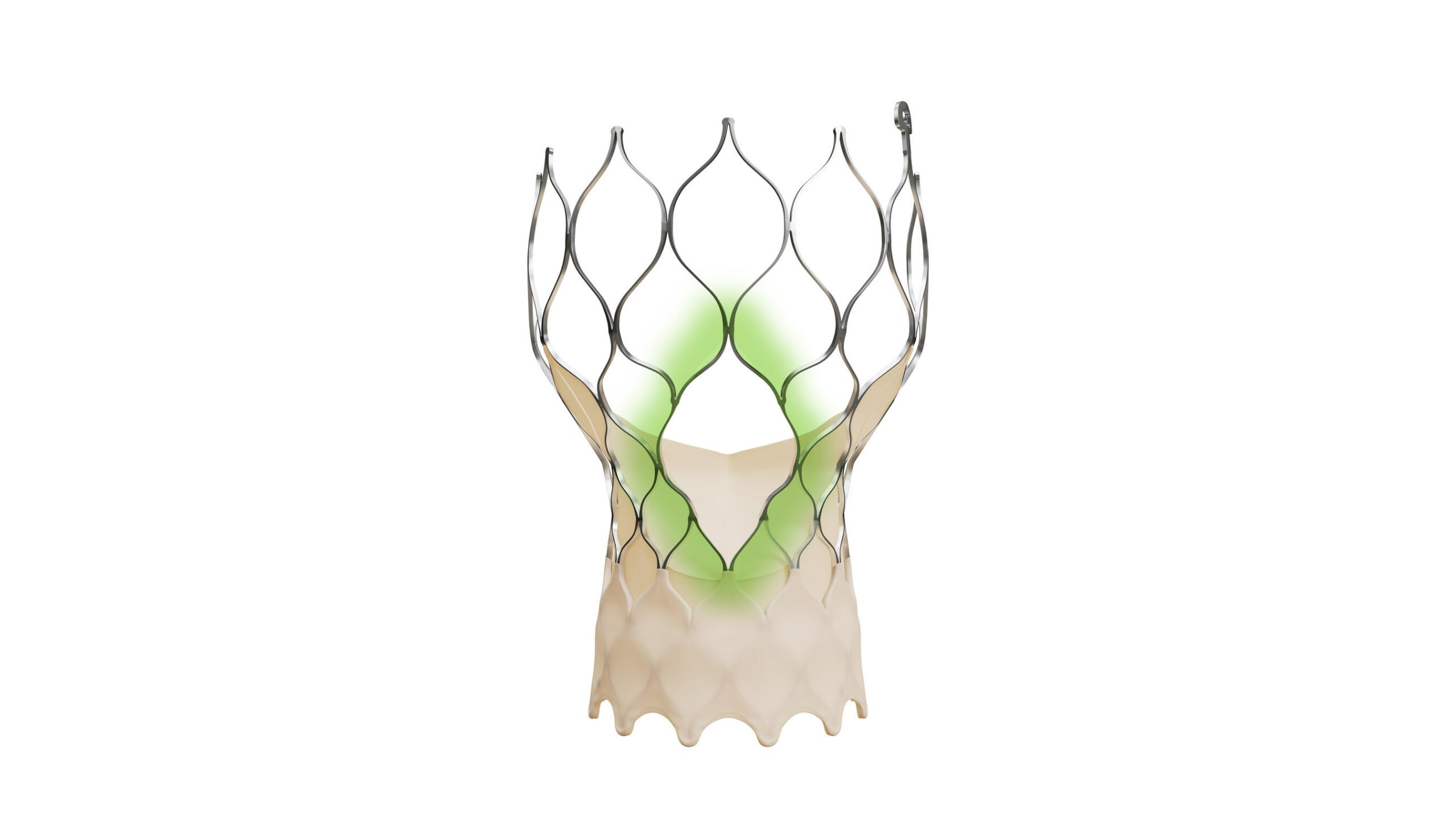
The new-generation TAVR system is designed to maintain the valve performance benefits of the legacy Evolut TAVR platform, and it facilitates coronary access.
It comes with a modified diamond-shaped frame design, which is four times larger than previous versions of the Evolut TAVR system and offers bigger coronary access windows.
The Evolut FX+ TAVR system provides more space for catheter manoeuvrability to facilitate access to coronary arteries of different patient anatomies.
In addition, the new design does not compromise the valve performance, haemodynamics, and radial strength clinicians desire from the Evolut platform, said the medical device maker.
Medtronic intends to launch the system initially under an Early Commercial Experience programme in the spring of this year, with plans for a full commercial launch in the summer.
Medtronic coronary and renal denervation business and the structural heart and aortic business vice president and chief medical officer Jeffrey Popma said: “We are committed to consistently developing and advancing minimally invasive solutions for physicians to treat their patients with aortic stenosis.
“This is reinforced by our continued innovation of the Evolut TAVR platform, which has delivered proven valve performance and durability to physicians and patients for years.
“The Evolut FX+ TAVR system was designed to facilitate coronary access across a diverse range of patient anatomies with no compromise to valve performance.”
In October last year, Medtronic announced four-year results from the Evolut Low Risk Trial, in which the TAVR system showed exceptional outcomes and sustained valve performance.
The Evolut TAVR has resulted in significantly better haemodynamics compared to surgical aortic valve replacement (SAVR).
Also, the system consistently lowered diverging rates of death or disabling stroke compared to advanced surgery, at four years.






