The move expands Stratasys’ suite of printers and materials validated by its collaborator Materialise as part of FDA-cleared Materialise Mimics inPrint software.
It brings a new 3D printing system for point-of-care across hospitals and physicians. Materialise has validated PolyJet multi-material and multi-color solutions including J750 and J735 3D Printers and Objet30 Prime 3D Printer.
The software is claimed to have been designed to allow physicians and hospitals to use 3D printing at point-of-care to build a trusted and reliable source for surgical planning and interdisciplinary communication.
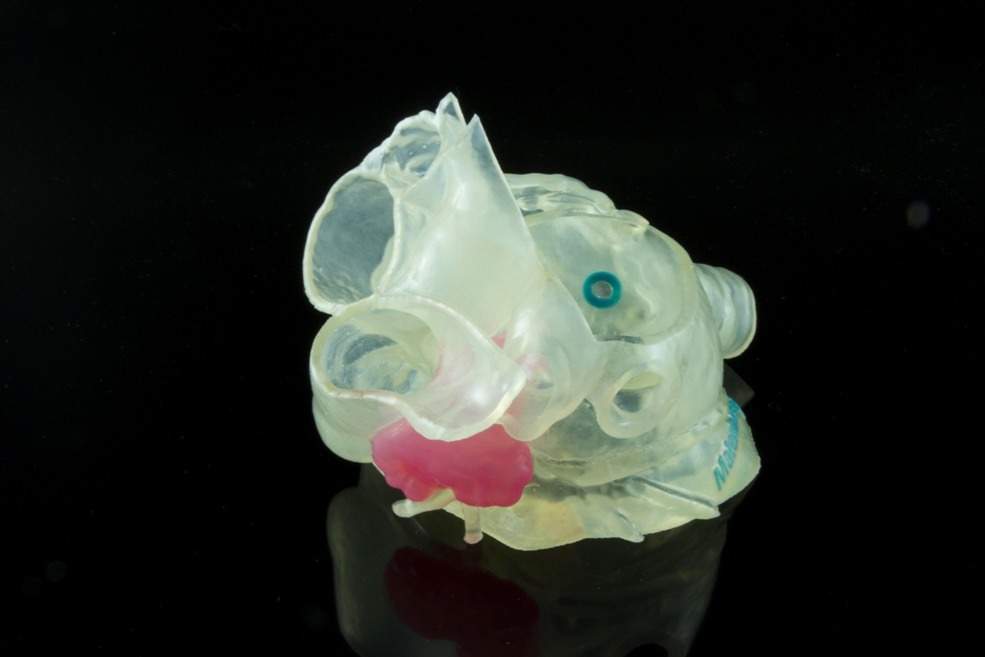
Stratasys said its J735/J750 3D Printers can develop complex models using multiple textures by combining hard and soft materials to mimic human tissue. This combination of transparency with multiple color re-creation can make sure that practitioners can differentiate anatomy, view critical structures within an organ replica and create realistic representations of any bone, tissue and organ.
The company’s Objet30 Prime 3D Printer is a cost-effective, desktop platform that offers an entry point for hospitals that seek a point-of-care printing solution, without compromising quality, resolution or accuracy.
The printer can support a range of anatomical models and applications, including orthopedic, cardiac, neurosurgery and other use-cases for visualization, surgical planning, training and education.
Stratasys healthcare business unit head Eyal Miller said: “Historically, pre-surgical planning relied on 2D imaging requiring physicians to mentally reconstruct the patient anatomy. But 3D printing evolves this approach by putting precise replicas of patient anatomy directly in physician hands. Our collaboration with Materialise is a huge step towards unlocking the potential of this technology for patient care.
“Now the 3D printer that every hospital needs to power their medical modeling comes with additional options for an FDA-cleared software solution.”
In March this year, Materialise Mimics inPrint secured FDA clearance and became the only solution with 510(k) clearance as an end-to-end 3D printing solution and a fully comprehensive 3D printing solution for anatomical modeling.
The company also claimed that 16 out of the top 20 US hospitals, ranked by US News & World Report, have implemented a medical 3D printing strategy using Materialise Mimics technology.
Materialise North America vice president and general manager Bryan Crutchfield said: “By validating Stratasys’ 3D printing technologies through our certification process, we’re giving doctors and hospitals improved access to high-quality anatomical models for personalized care to patients.”






