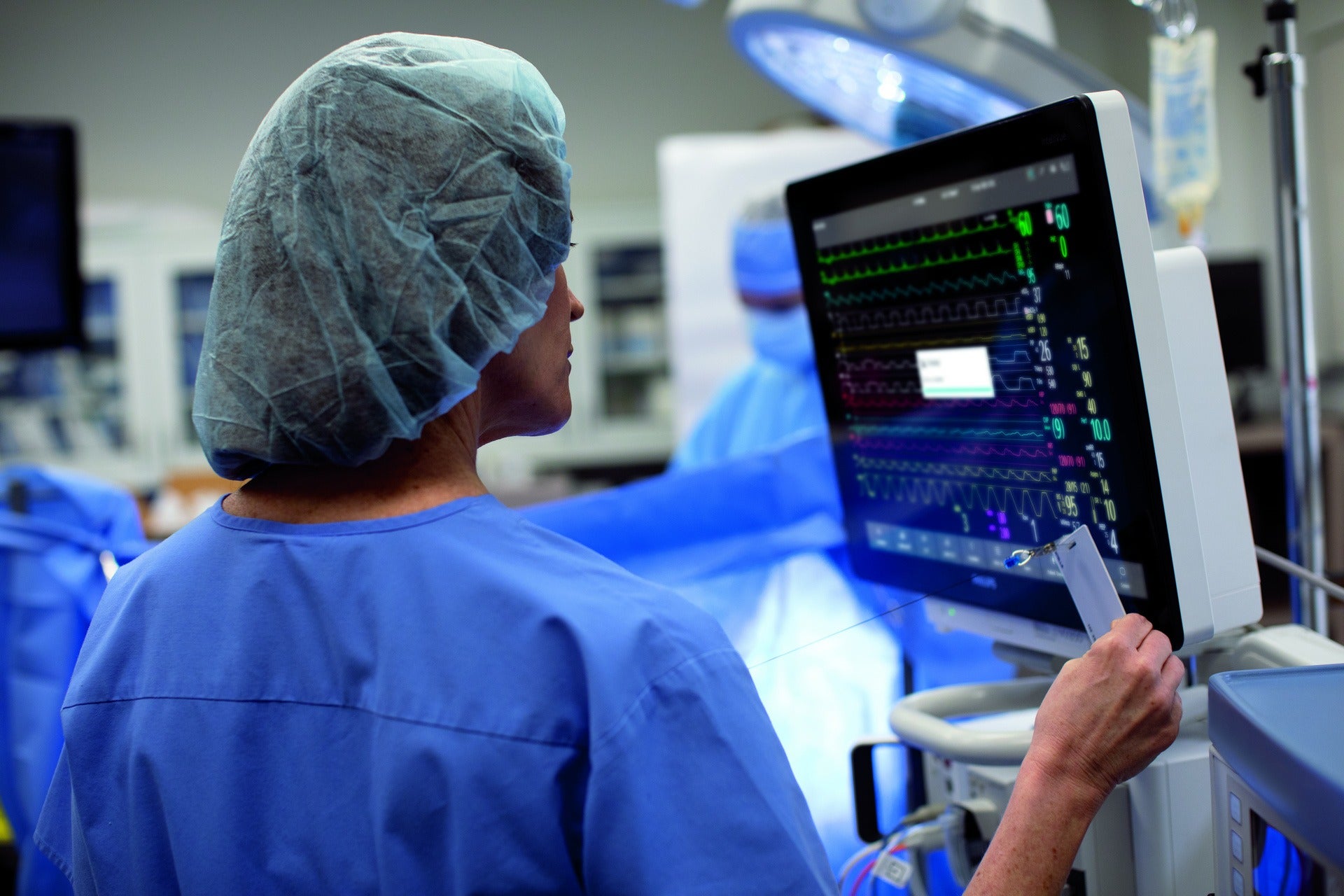
Royal Philips and Masimo announced that the US Food and Drug Administration (FDA) has approved incorporation of Masimo’s advanced monitoring technologies into Philips high acuity patient monitors.
The FDA approval allows integration of Masimo’s SedLine Brain Function Monitoring, Regional Oximetry (O3), and CO₂ measurements into Philips’ IntelliVue MX750 and MX850 patient monitors.
The integration will extend ongoing partnership between Masimo and Philips, and help clinicians rapidly make informed decisions, without needing additional monitoring equipment.
It enables clinicians to monitor blood saturation in the brain, anaesthetic sedation, and patient respiratory performance, and allows for data sharing between monitors, said Philips.
Philips hospital patient monitoring general manager Christoph Pedain said: “Our work with Masimo has enabled us to forge new paths in continuous monitoring.
“We’re connecting data and technologies to help arm care providers with the robust information they need to make timely, informed care decisions for their patients.”
In November 2016, the Dutch health technology firm and the US-based medical technology company have initially partnered to focus on patient monitoring and therapy solutions.
Earlier this year, the companies expanded their collaboration to boost patient monitoring capabilities in home telehealth applications with the Masimo W1 health tracking watch.
Since the beginning of their partnership, Philips and Masimo have introduced several advanced monitoring capabilities to IntelliVue MX-series patient monitors.
The FDA approval indicates the expansion of O3 regional oximetry capability to Philips’ high acuity MX750 and MX850 patient monitors.
Also, it implies addition of new SedLine Brain Function Monitoring and CO₂ measurement capabilities to Philips’ portfolio patient monitoring solutions.
Masimo worldwide OEM sales and global health president Jon Coleman said: “Combining our expertise in non-invasive monitoring and signal processing technologies with Philips’ expertise in integrated patient monitoring and therapy solutions is a win-win for patients and clinicians alike.
“We are proud that Philips has chosen to make our innovative SedLine, O3, and NomoLine technologies available to their customers. We look forward to continuing our partnership with a focus on improving patient outcomes and reducing the cost of care.”



