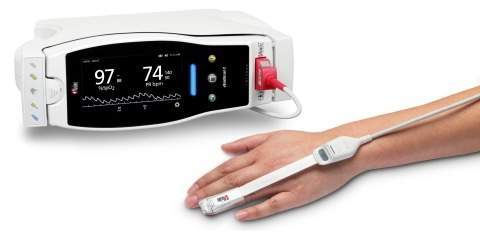
The RD SET sensors with Masimo measure-through motion and low perfusion SET pulse oximetry have secured FDA clearance with improved SpO2 accuracy specifications for all patients above 3kg.
According to the company, the new RD SET sensors’ SpO2 accuracy specifications during patient motion have improved for adult, pediatric, and infant patients to 1.5% compared against previous accuracy specifications of 3%.
Featuring an advanced sensor-to-cable connection, the sensors are lightweight and their recyclable packaging enables to reduce storage and shipping space.
The studies have demonstrated that Masimo SET allowed to reduce rates of severe retinopathy of prematurity (ROP) and the requirement for laser treatment and enabled pulse oximetry screening for critical congenital heart disease (CCHD).
The use of Masimo SET allowed to reduce rescue calls and ICU transfers by 65% and 48% on post-surgical wards.
In addition, the studies showed that post-operative continuous surveillance monitoring, combined with Masimo SET, led to zero preventable deaths or brain damage due to opioids over five years.
Masimo founder and CEO Joe Kiani said: “We’re delighted to be able to announce our continued innovation in our foundational SET pulse oximetry. Thanks to the brilliance and dedication of our engineers and the continuing support of our customers, we’ve been able to once again raise the standard for pulse oximetry performance.
“Even though no one has been able to create pulse oximetry that outperforms SET, we have not allowed that to stop us from continuing our pursuit of perfecting the technology.”
In September this year, The FDA has approved asimo’s RAS-45, an acoustic respiration sensor for rainbow Acoustic Monitoring (RAM), for infant and neonatal patients.
Earlier, only patients over 10 kilograms were eligible for RAM sensors, but with the new clearance acoustic respiration rate measurement is now possible for patients of all sizes, including neonates, in the US.
Masimo provides advanced noninvasive monitoring technologies to enhance patient outcomes and reduce the cost of care.


