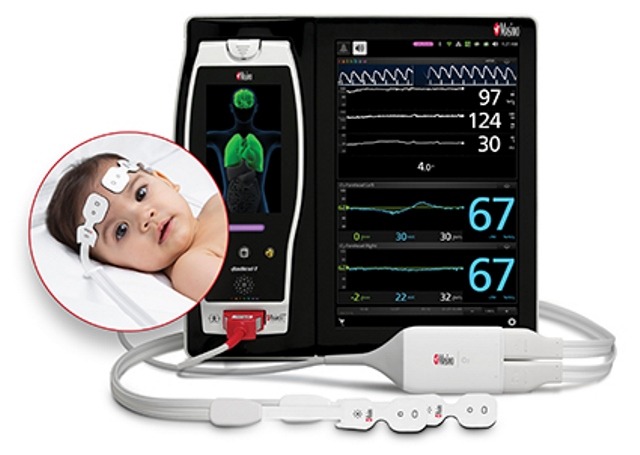
O3 regional oximetry will allow clinicians to monitor cerebral oxygenation in conditions where peripheral pulse oximetry alone may not be fully indicative of the oxygen in the brain.
The current approval enables the company to provide O3 regional oximetry to monitor patients of all ages ranging from neonates to adults.
Masimo’s O3 utilizes near-infrared spectroscopy (NIRS) to monitor the regional hemoglobin oxygen saturation of blood (rSO2) on both sides of the brain. It is more useful in offering insight into neonatal patient status, as neonatal pathology is mostly brain-related.
According to the company, O3 provides reliable measurement with a 3% ARMS trending accuracy specification in neonates.
The company is offering O3 sensors in three sizes, which can be used for adult (≥40 kg), pediatric (≥5 kg and <40 kg) and infant and neonatal (<10 kg) patients.
Due to its smaller size and flexible design, the neonatal sensor will fit easily and comfortably on the delicate foreheads of tiny patients.
The company is providing O3 as a Masimo Open Connect (MOC-9) module for the Root patient monitoring and connectivity platform.
Root is an expandable hub, which incorporates an array of technologies, devices, and systems to offer multimodal monitoring and connectivity solutions.
The plug-and-play expansion capabilities of Root will enable clinicians to simultaneously monitor with O3 and other measurements such as SET Measure-through Motion and Low Perfusion pulse oximetry, helping clinicians to know neonatal oxygenation status.
Root also integrates additional modalities, including advanced rainbow noninvasive measurements such as total hemoglobin (SpHb), SedLine brain function monitoring and NomoLine capnography.
Root, along with Masimo Patient SafetyNet or Iris Gateway, can be used to automatically monitor and charted data from O3 in electronic medical records (EMRs).
Masimo founder and CEO Joe Kiani said: “Our foundational SET pulse oximetry was designed with neonates and infants in mind. These patients were not an afterthought.
“This focus has paid off for these young patients: SET pulse oximetry has helped clinicians reduce the incidence of severe retinopathy of prematurity (ROP) in neonates2 and improve critical congenital heart disease (CCHD) screening in newborns.”



