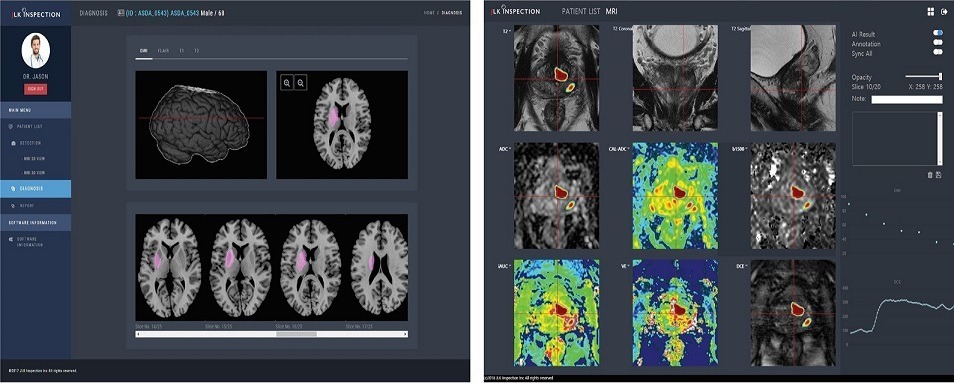
JPC-01K is an AI-based prostate MR image analysis solution, while JBS-01K is an AI-based brain image analysis solution.
JPC-01K is an AI medical system that detects the area of prostate cancer using multiparametric MR images. The system is based on artificial neural networks to analyze the MR images of the prostate cancer patients. It uses multiparametric MR images, T2, DWI, and DCE to visualize the location of the prostate cancer and their probability.
JBS-01K is an AI medical system, which diagnoses a subtype of ischemic stroke using patient’s MR image and atrial fibrillation (AF) information.
The system carries out lesion detection and TOAST (trial of ORG 10172 in acute stroke treatment) classification of ischemic stroke using 3D hybrid artificial neural networks.
JBS-01K offers classification probabilities by analyzing input MR images of 4 sequences (DWI, FLAIR, T1, T2) and clinical information data.
In 2018, JLK Inspection introduced an all-in-one medical platform, AIHuB, which comprises 37 medical solutions, including JPC-01K and JBS-01K.
AIHuB holds capacity to detect and monitor 37 medical conditions in 14 different regions of the body.
The platform can also carry out analysis based on multimodal images, including MRI, CT, X-ray, and mammogram images, using an AI-enabled technology that encompasses a range of techniques for diagnosing illnesses, such as stroke, Alzheimer’s, and cancer.
To enter the global market, the company intends to actively participate in various international exhibitions and conferences, including Radiological Society of North America (RSNA) 2019, European Congress of Radiology (ECR) 2019 and Consumer Electronics Show (CES) 2020.
JLK Inspection representative said: “It is noteworthy that our solutions have been acknowledged as products that meet world-class quality standards.”
“Starting with CE certification, we are planning to obtain FDA approval and expand our market to the United States in the foreseeable future.”
Based in South Korea, JLK Inspection has offices in Silicon Valley, California as well as research and development (R&D) and manufacturing sites in Seoul, South Korea.



