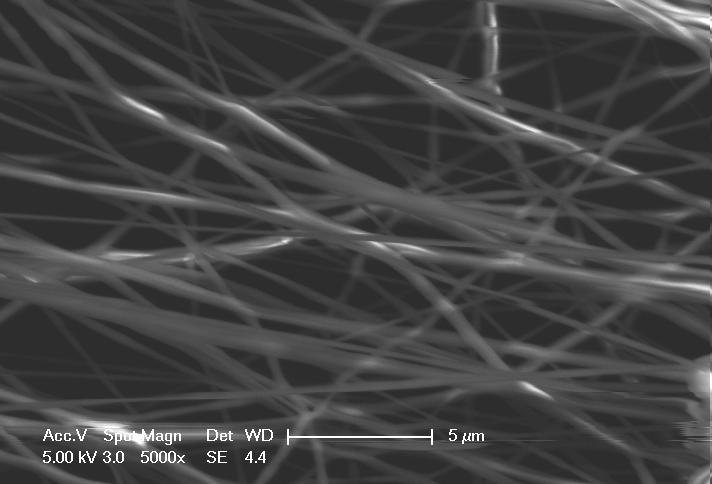
IME said that the new platform marks an innovation in the controlled production of well-defined scaffolds for implants and membranes on a large scale, using fibres ranging from nanometer up to micrometer scale.
The MediSpin XL platform is expected to strengthen the company’s global standard for the joint development and production of scalable and reproducible nanometer and micrometer scaffolds.
IME has applied specific polymers and its advanced equipment created fibre-based medical device solutions that mimic the natural human extracellular matrix in nanometer and micrometer format for implants and membranes in the human body.
In contrast to implants and membranes of traditional structures, which are seen as foreign bodies and lead to rejection phenomena, the new solution makes human cells recognise the artificial matrix scaffold as the body’s own and allows the damaged tissue of various organs to be repaired.
IME Medical Electrospinning managing director Judith Heikoop said: “The global launch of our state of the art production platform is testimony to our strong belief in the strategic goal of becoming the leading developer and producer of the most advanced electrospinning equipment for both large-scale production and R&D purpose, and broadens our trusted partnership worldwide in co-developing electrospun medical devices, which are seen as true game changers in the MedTech industry.”
The company has designed MediSpin XL platform specifically for MedTech industrial manufacturing of medical devices to ensure control of the crucial parameters leading to identical and consistent end-products.
Crucial parameters include fibre diameter and structure, porosity, mesh thickness and tensile strength, accompanied by changes in temperature and relative humidity between the seasons and during the day, which are vital for the in-vitro and in-vivo functionality of products, and could lead to inconsistencies if not controlled.
IME claims that the MediSpin XL platform marks the first large-scale production platform that prevents all the factors that might distort the electrospinning process, along with enabling an optimal process set-up and stability, including climate control and on-line quality monitoring measurements.
IME Medical Electrospinning founder and managing director Ramon Solberg said: “This technology enables the large-scale manufacturing of reproducible and scalable fiber-based scaffolds, the wonderful pieces of art that will substantially transform the medical device market for a wide variety of medical applications and thus revolutionize regenerative medicine.”




