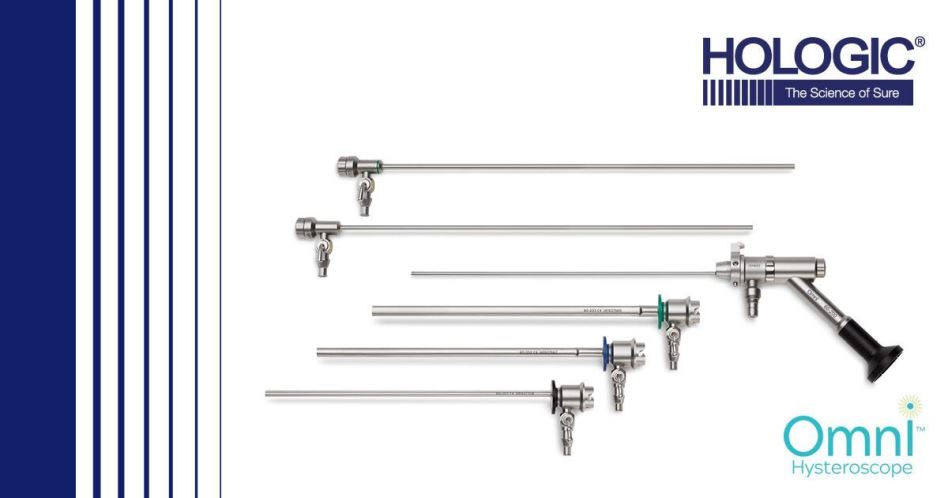
Hologic said the Omni Hysteroscope has been designed to help Obstetricians and gynecologists (ObGyns) for both in- and out-patient settings and help ObGyns simplify the process of diagnosing and treating patients.
Often times, surgeons require a diagnostic scope to look in the uterine cavity for fibroids or polyps, then switches to an operative scope to biopsy or treat the pathology. The new Omini scope allows ObGyns to use the same scope with different sheath options to conveniently see and treat pathology.
Hologic Omni scope features MyoSure optics for quality, visualization and clarity throughout the procedure. The sheaths have been designed with smaller diameters including 3.7 mm diagnostic sheath, 5.5 mm operative sheath, 6 mm operative sheath.
They are claimed to reduce dilation needed to promote patient comfort and easy insertion. The sheaths can also assembled and disassembled easily between procedures.
The 200mm working length offers easy access and treatment for obese patients. The Omni scope is compatible with MyoSure tissue removal offerings including MyoSure REACH, MyoSure XL, MyoSure LITE, and MyoSure MANUAL devices.
Hologic medical director and global medical affairs vice president Edward Evantash said: “Experts agree that direct visualization of the uterine cavity in women with abnormal uterine bleeding is the gold standard that allows ObGyns to accurately identify and collect quality samples and remove pathology – in a safer and more effective manner than blind biopsy and curettage.
“Featuring three easily interchangeable sheaths in one scope, our new Omni scope gives ObGyns excellent visualization capabilities with the convenience of seeing and treating pathology in a more streamlined procedure.”
Hologic EMEA and Canada regional president Jan Verstreken said: “We continue to drive innovation designed with both our customers and the women they treat in mind. Our customers are under pressure to deliver high-quality care to their patients in innovative ways. Our new Omni Hysteroscope provides them with a pioneering device that can treat a diverse range of patients.”
Besides receiving CE Mark approval in Europe, the company also received medical device license for the Omni Hysteroscope from Health Canada, the government health department in the country.






