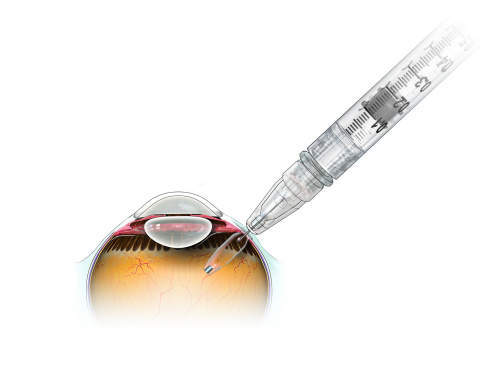
The small refillable eye implant, which slightly longer than a grain of rice, has been designed to allow people with wet AMD to go several months without requiring to visit their ophthalmologist for treatment.
According to the company, vision outcomes in the high-dose PDS group were similar to monthly ranibizumab eye injections and were maintained across the study period.
LADDER study investigator for Dr Carl Regillo said: “I believe that more consistent treatment could allow for better long-term vision outcomes in clinical practice.”
LADDER (long acting delivery of ranibizumab; NCT02510794) is a phase II study designed to assess the efficacy and safety of the port delivery system with ranibizumab in people with AMD who have previously responded to treatment with anti-vascular endothelial growth factor (VEGF) therapies.
The multicenter, randomized, interventional and active treatment-controlled study recruited 243 participants in around 50 sites across the US.
The trial patients implanted with the PDS secured one of three concentrations of ranibizumab, including 10mg/mL, 40 mg/mL or 100 mg/mL.
Primary objective of study was to determine the time until a patient first needed a refill of the implant, evaluating the efficacy and safety of three different concentrations of ranibizumab compared to monthly intravitreal injections of ranibizumab 0.5mg.
Secondary endpoints of the study comprised of assessments of vision and anatomic outcomes when compared to monthly intravitreal ranibizumab 0.5 mg injections.
Lucentis (ranibizumab injection) is a VEGF) designed to bind to and inhibit VEGF-A, a protein that is expected to play a crucial role in the formation of new blood vessels (angiogenesis) and the hyperpermeability (leakiness) of the vessels.
Genentech chief medical officer and global product development head Dr Sandra Horning said: “LADDER is the first successful Phase II study of a long-acting delivery device for the treatment of wet AMD. We are highly encouraged by these results and the potential of the PDS, our first implantable, drug delivery program.
“With the PDS, we have an opportunity to make a positive impact by helping to potentially eliminate the burden of frequent treatments for people with wet AMD.”






