RheOx is a bronchoscopic system that will help reduce mucus-producing cells in patients with chronic bronchitis
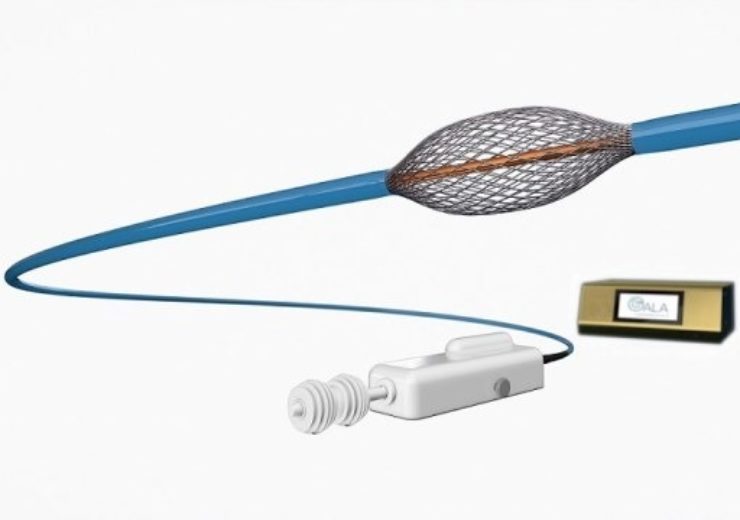
Image: Gala Therapeutics' RheOx Bronchial Rheoplasty system. Photo: courtesy of PRNewswire / Gala Therapeutics, Inc.
Gala Therapeutics has secured a breakthrough device designation (FDA) from the US Food and Drug Administration (FDA) for its RheOx bronchial rheoplasty system.
Through using a minimally invasive procedure, the RheOx system will provide non-thermal energy to the airways to minimise mucus-producing cells in patients with chronic bronchitis.
Gala Therapeutics’ investigational RheOx bronchial rheoplasty system
RheOx features an advanced technology, which includes an electrosurgical generator and single-use catheter that together provide non-thermal energy to the airways to reduce the number of abnormal mucus-producing cells in the lungs, helping to create new way to redevelop new normal cells.
The system, which is presently under assessment in an early feasibility study in the US, is limited by federal law to investigational use. Earlier this month, the company also secured CE mark approval for the RheOx bronchial rheoplasty system.
According to the company, chronic bronchitis affects more than nine million people in the US and occurs in people with chronic obstructive pulmonary disease (COPD) and individuals with normal lung function.
Gala Therapeutics CEO Jonathan Waldstreicher said: “Today there are no treatments to address the debilitating symptoms impacting the quality of life in patients with Chronic Bronchitis. We are excited by FDA’s decision to designate RheOx as a breakthrough device, underscoring the significant unmet need for these patients.
“We look forward to working with FDA to bring a solution that improves the lives of these patients.”
In September 2018, Gala Therapeutics recruited first two patients in the early feasibility study of RheOx for chronic bronchitis, and were treated at University of Pittsburgh Medical Center (UPMC) in Pittsburgh, Pennsylvania.
Based in Menlo Park of California, Gala Therapeutics is involved in the development of disease-modifying therapies to treat patients with pulmonary diseases.
Established by Apple Tree Partners, the company is building advanced technologies to help interventional pulmonologists, thoracic surgeons and physicians to treat pulmonary disease.
