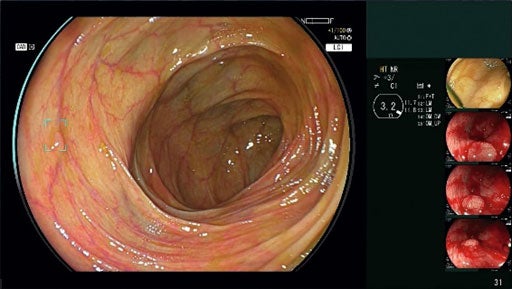
FUJIFILM Healthcare Americas has received the US Food and Drug Administration (FDA) 510(k) approval for its new AI-powered detection endoscopic imaging system, CAD EYE.
CAD EYE is designed to facilitate real-time detection of colonic mucosal lesions such as polyps and adenomas during colonoscopy procedures.
The system also supports endoscopists in detecting and removing pre-cancerous lesions, irrespective of their size, shape, and colour, said the company.
It is an advanced version of Fujifilm’s ELUXEO Endoscopic Imaging System and features the Fujifilm EX-1 expansion unit and EW10-EC02 endoscopy support software.
CAD EYE also features an AI image processing functionality, personalized for the integration with the system’s processor and the endoscope.
Fujifilm developed the system at its Tokyo-based global AI technology centre, using deep learning technology, and validated it in several studies using polyps in clinical images.
FUJIFILM Healthcare Americas endoscopy general manager Tai Fujita said: “Colorectal cancer is the second leading cause of cancer deaths in U.S. women and men combined, despite screening, primarily colonoscopy, being one of the most reliable and effective methods for cancer prevention and early intervention.
“Every day we pride ourselves on delivering what we believe is the highest quality imaging and optics, arming endoscopists with the tools they need to combat this public health issue.
“Today, we’re thrilled to take that a step further with the introduction of CAD EYE, which has the potential to dramatically improve the quality of colonoscopy.”
According to clinical studies, CAD EYE may lead to considerable advances in the detection and diagnosis of colorectal cancer.
The system detects more adenomas during screening compared to conventional high-definition colonoscopy without AI assistance, and without extending procedure time.
CAD EYE results in the detection of 17% higher adenoma per colonoscopy (APC) compared to high-definition conventional colonoscopy.
Also, it detects colorectal neoplastic lesions at comparable levels to that of an expert and superior to that of a beginner.
Furthermore, CAD EYE can be used with both White Light Imaging, and Linked Color Imaging (LCI), Fujifilm’s enhanced visualisation mode enhances mucosal visualisation.
In a separate development, FUJIFILM India, a fully owned subsidiary of the Japanese company, has introduced the ALOKA ARIETTA 850 diagnostic ultrasound system in India.
The company has installed the first-ever system at Fortis Hospital in Bengaluru, Karnataka.
The ALOKA ARIETTA 850 system at Fortis Hospital will provide advanced diagnostic and therapeutic services for patients against GI-related diseases.
It also allows performing targeted treatments such as Pancreatic pseudo cyst drainage without surgery, and targeted delivery of treatment in Pancreatic cancers.
Unlike conventional Endoscopic ultrasound machines, its ALOKA ARIETTA 850 system generates superior image quality with seven million digital channels, said FUJIFILM India.


