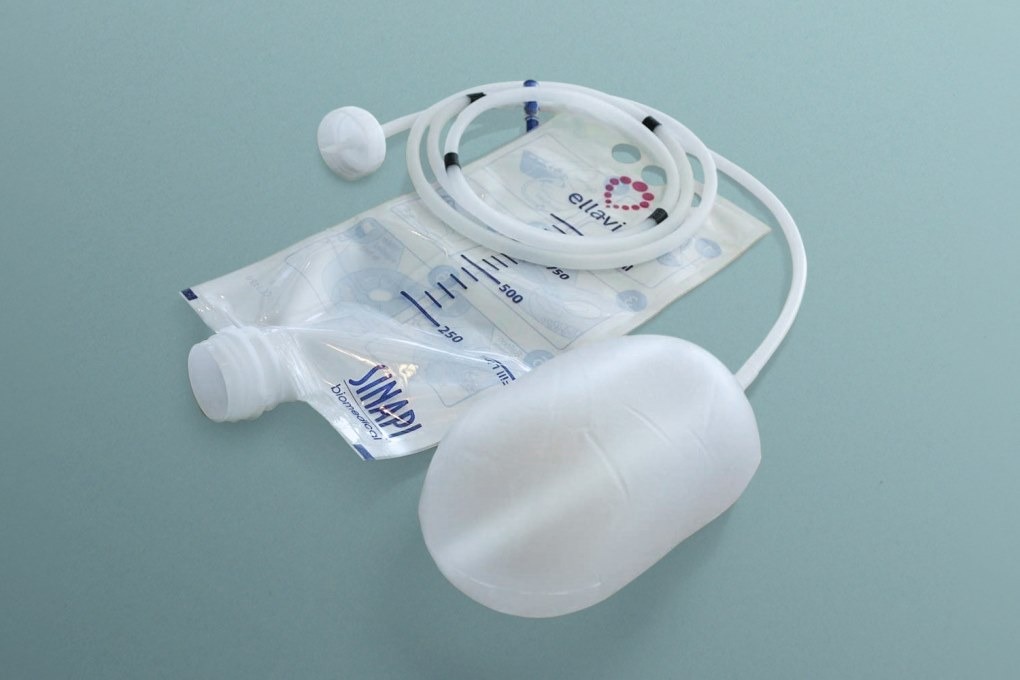
PATH and Sinapi Biomedical said that Ellavi UBT is a low-cost, fully assembled device, which is ready to be sold across Europe. Obtaining CE Marking is touted to be an important step in the mainstream use of this lifesaving emergency intervention in countries with high maternal mortality rates.
Across many developing countries, such as the sub-Saharan Africa, CE Marking can help in the device’s procurement.
Excessive bleeding after childbirth or severe postpartum hemorrhage (PPH) is claimed to be the number one killer of women during childbirth and can kill an otherwise healthy women in less than two hours, if the required care is not given to her.
The World Health Organization (WHO) recommendations identify, UBT as an emergency intervention for PPH when drug treatment fails or is unavailable.
However, UBT products have been in use in hospitals for decades in high-resource countries and cost hundreds of dollar each, thus they have been out of reach of many health facilities in low-resource countries.
Instead, these health facilities rely on improvised balloon tamponades that are assembled on the spot with supplies such as a male condom or latex glove and a catheter pieced together with a string. The gap in access is significant, as developing countries account for nearly 99% of all maternal deaths.
PATH UBT product manager Elizabeth Abu-Haydar said: “PPH can happen to any pregnant woman, anywhere in the world. These deaths can be prevented; however, the mothers who are most likely to die from PPH are those living in sub-Saharan Africa, reflecting the inequities in access to health care and appropriate technologies.
“We championed an innovative solution that could prevent the deaths of thousands of pregnant women—a low-cost UBT that performs to the same high standards as those used to save lives in high-resource hospitals, yet is affordable and preassembled for ease of use in health facilities in resource-poor communities.”
PATH and Sinapi Biomedical developed the Ellavi UBT by taking input from physicians, midwives and maternal health experts. Once the Ellavi balloon is placed in the uterus by a health care provider, the balloon fills in less than one minute with water from a supply bag.
Sinapi Biomedical managing director Chris De Villiers said: “Unique design innovations include our pressure-controlled, free-flow system that applies optimal pressure to the uterine wall to arrest bleeding in minutes, allowing the woman’s uterus to contract and relax when its normal function is restored.
“We designed Ellavi to be intuitive to use by providers during the most critical moments of their job.”






