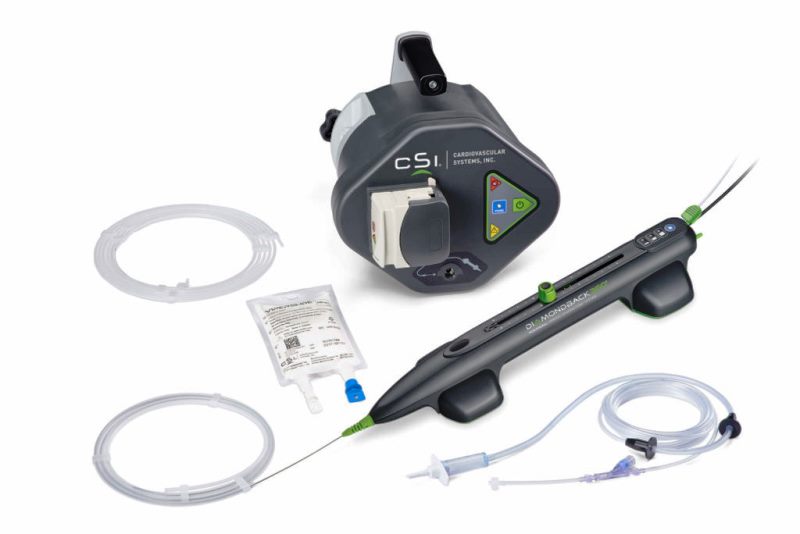
According to CSI, REACH PVI is a prospective, observational, single-arm, multi-centre post-market study and is aimed at evaluating the acute clinical outcomes of orbital atherectomy through transradial access for the treatment of peripheral artery disease (PAD) in lower extremity lesions.
The company’s low profile, 5Fr, Extended Length Diamondback 360 Peripheral Orbital Atherectomy System (OAS) and Ehttps://investors.csi360.com/news-releases/press-release-details/2019/Cardiovascular-Systems-Inc-Announces-First-Patient-Enrolled-in-REACH-PVI-Clinical-Study/default.aspxxtended Length Stealth 360 Peripheral OAS are the atherectomy devices that allow radial access for the treatment of peripheral lesions.
The national primary investigator for the REACH PVI study, Dr. Ankur Lodha, who is an interventional cardiologist at Cardiovascular Institute of the South, Lafayette, Louisiana, enrolled the first patient.
Dr. Lodha said: “When endovascular intervention is necessary, PAD lesions are mostly treated through femoral artery access. However, many PAD patients could benefit from faster ambulation time post procedure that radial artery access offers. In addition, some patients may have comorbidities, such as obesity, that complicate or preclude femoral access.
“The benefits of radial access for percutaneous peripheral vascular interventions are well documented. We believe this study will demonstrate that many of these known benefits, such as low complication rates, high cost effectiveness, and short time to ambulation, can be achieved using orbital atherectomy to treat lower limb PAD lesions through radial access.”
The study involves enrolment of 50 patients at 10 sites across the US, and includes CSI orbital atherectomy devices FDA-cleared for treatment of PAD.
All the subjects of the study are expected to be followed post-procedure through the first standard of care follow-up visit between 7-45 days after the procedure.
In August 2007, the company has secured the US FDA 510(k) clearance for the use of the Diamondback Orbital Atherectomy System in peripheral arteries, followed by the FDA approval for use of the Diamondback Orbital Atherectomy System in coronary arteries in October 2013.
CSI chairman, president, and chief executive officer Scott Ward said: “CSI continues to innovate and develop medical evidence to improve the care of patients suffering from PAD. The commercialization of the Extended Length orbital atherectomy systems and our investment in REACH PVI both demonstrate our commitment to physicians and the patients they serve.”
CSI is engaged in developing and commercializing innovative interventional treatment systems for patients with peripheral and coronary artery disease.






