The Canada-based company designed the system components to help in the evaluation of patients at point-of-care for cardiac disease using a static machine-learned detection algorithm
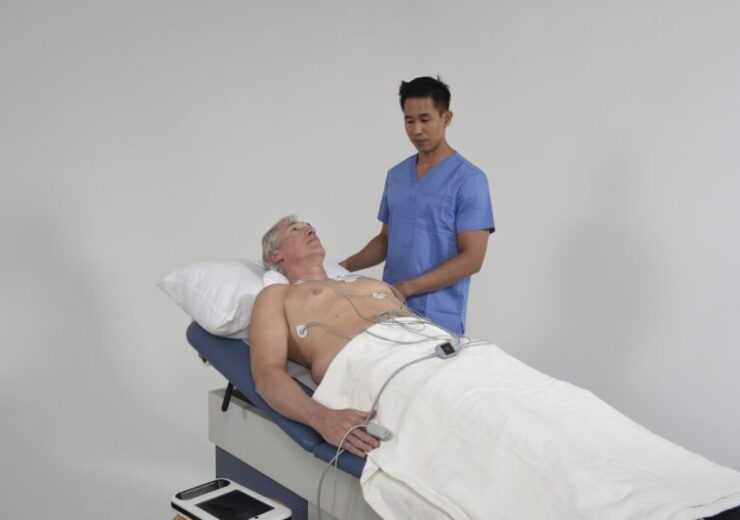
The CorVista System is a non-invasive point-of-care solution. (Credit: Business Wire)
Digital health company CorVista Health has received the US Food and Drug Administration (FDA) clearance for its CorVista System with CAD Add-On to identify cardiac diseases.
The CorVista System is a non-invasive medical device system made up of hardware and software components. It is intended for the detection of major coronary artery disease to support the diagnosis of cardiovascular disease.
CorVista Health has designed the system components to help in the evaluation of patients at point-of-care for cardiac disease using a static machine-learned detection algorithm.
The CAD Add-On is said to be the Canada-based digital health firm’s planned suite of cardiac detection algorithms to have obtained market clearance.
The CorVista System is intended to assess sensor-acquired physiological signals from patients with cardiovascular symptoms like chest discomfort, dyspnea, or fatigue to determine the risk of coronary artery disease.
CorVista Health, which was previously operating as Analytics 4 Life, the analysis is interpreted by healthcare professionals as a diagnostic tool together with their clinical expertise, the patient’s signs, symptoms, and clinical history.
CorVista Health president and CEO Don Crawford said: “From all of us at CorVista Health, we are thrilled to announce we have obtained FDA clearance of the CorVista System.
“Our point-of-care, non-invasive solution rules out significant CAD with a negative predictive value (NPV) of 99%. Furthermore, it requires no fasting, radiation, or significant capital investment.
“The ability to obtain a result within minutes will be a game changer in aiding physicians to detect the potential presence of cardiac disease.”
The FDA clearance was based on a blinded clinical validation dataset gathered as part of the IDENTIFY trial. The results showed performance in diagnosing severe CAD with sensitivity of 88% and specificity of 51%.
These outcomes can be compared to the rule-out performance of coronary computed tomography angiography (CCTA), the digital health company said. Additionally, the underserved female population continues to exhibit the same performance.
This clearance comes after the company’s second disease-specific Add-On module for pulmonary hypertension was announced to have received the FDA Breakthrough Designation.
