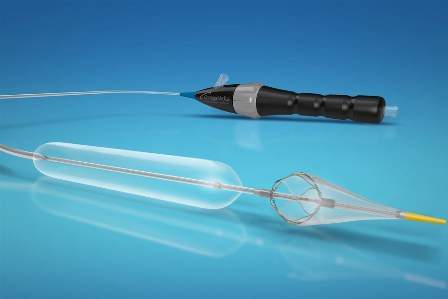
The Vanguard IEP system integrates a peripheral angioplasty balloon and distal embolic filter on the same catheter.
The device has been developed to safeguard the lower limbs during angioplasty without the support of additional devices or exchanges.
Contego Medical founder and CEO Dr Ravish Sachar said: “Securing FDA clearance for Vanguard IEP represents a major milestone for Contego Medical.
“As the patient population with peripheral arterial disease continues to expand and become more complex, we believe our technology will play a critical role in protecting vulnerable patients from embolic events and thus improve procedural outcomes.”
The Vanguard IEP peripheral balloon angioplasty system with integrated embolic protection can be used for percutaneous transluminal angioplasty (PTA), capture and removal of embolic material during angioplasty, as well as for use in the renal, cerebral, coronary or carotid vasculature.
It features an over-the-wire design with a sheathless incorporated 150μ pore filter distal to the angioplasty balloon.
Contego Medical’s filter is claimed to be the first to have in-vivo adjustability for use with varying vessel sizes and enhance capture efficiency.
In Europe, the Entrap 112-patient post-market registry assessed the Vanguard IEP system, in which all the patients achieved primary safety and efficacy endpoints at discharge and 30 days.
The Entrap study principal investigator professor Thomas Zeller said: “The Vanguard IEP System is designed to protect patients at high risk for embolization during peripheral angioplasty, such as those with acute limb ischemia, severe calcification, and chronic total occlusions if crossed intraluminally.”
Contego Medical is engaged in the development and commercialization of next-generation devices for in neurovascular, coronary and peripheral vascular disease applications.
The company currently produces two FDA-cleared devices, which can be used in interventional procedures.
Contego’s integrated embolic protection platform aggregates embolic protection and treatment into one device to simplify catheter-based procedures and enhance patient outcomes.






