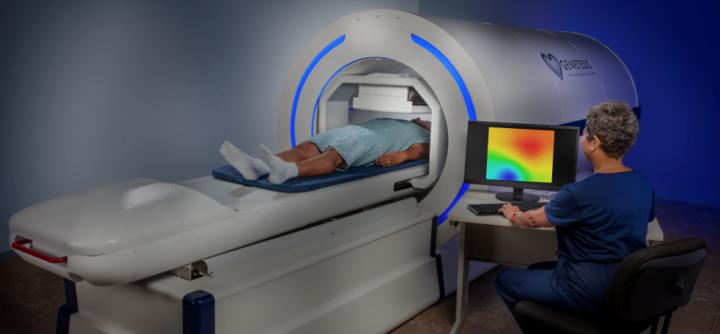
Genetesis said that its cardiac imaging platform works by pairing the CardioFlux Magnetocardiograph with the integrated Faraday Analytical Cloud (FAC).
CardioFlux technology allows simple, rapid, non-invasive cardiac imaging.
The CardioFlux’s use of optically pumped magnetometers (OPM) removes the need for liquid helium cooling, previously an obstacle to widescale commercial adoption of magnetocardiography (MCG).
Genetesis co-founder and CEO Peeyush Shrivastava said: “This milestone provides emergency room physicians and cardiologists with access to new technology to measure and visualize the magnetic fields produced by the heart’s natural electrical activity.”
CardioFlux data is transmitted securely to the Faraday Analytical Cloud, and physicians are allowed to review the functional imaging data, access a comprehensive set of web services from cloud, and hospitals leverage an integrated billing service.
St. John Hospital’s emergency department clinical decision unit medical director Margarita Pena has presented the investigational study carried out at Ascension observation unit using the prototype CardioFlux device, as a plenary session of the American College Emergency Physicians (ACEP) 2018 Scientific Assembly.
St. John Hospital Ascension cardiac catheterization labortaory director Edouard Daher said: “Building on the initial investigational study at Ascension St. John Hospital, we see the value in magnetocardiography (MCG) along with the 20 years of clinical investigation on the use of MCG and the diagnosis of myocardial ischemia and coronary artery disease.
“Ascension St. John is now collaborating with several sites to launch the largest multi-center study using MCG, to date.”
Pena said that the study has showed that there is great potential for magnetocardiography and CardioFlux would positively impact the clinical workflow of patients presenting to the ED with chest pain or anginal equivalents, which represent nearly 10 million emergency room visits a year.
West Virginia University heart and vascular institute cardiology division chief and JW Ruby Memorial Hospital cardiovascular imaging director Partho Sengupta said: “At WVU we see the potential impact of the use of magnetocardiography and CardioFlux and are in the process of implementing the CardioFlux system to conduct a study on the broader applicability to cardiovascular and coronary artery disease.”
Genetesis board of directors’ chairman and CincyTech life sciences director John Rice said: “These are major steps forward by the team at Genetesis. Achieving these clinical and commercial milestones enables delivery of an end-to-end medical device and cloud computing platform focused on improving patient care and reducing costs.”






