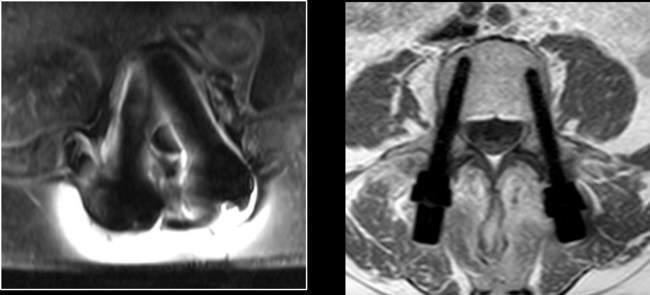
Earlier this year, the FDA approved the carbon fiber pedicle screw system of CarboFix to surgically treat oncological patients.
The benefits offered by carbon fiber implants to oncological patients and their physicians include enhanced radiation therapy planning abilities, enabling radiation treatment to optimize the radiation dose to the tumor with minimal collateral tissue damage and enhanced follow up abilities due to artifacts-free CT/MRI.
The implants also provide advanced fatigue strength to support the impaired healing process, and are compatible with particle radiation and other stereotactic radio-surgery modalities.
St. David’s Round Rock Medical Center neurosurgery director Carl Lauryssen was one of the first users of the CarboClear system in the US.
Lauryssen said: “These unique implants provide enhanced imaging follow-up and radiation treatment. These implants are a game changer for spinal oncology cases.”
CarboFix is engaged in the development, manufacturing and commercialization of advanced orthopedic solutions.
The firm’s products are approved by the FDA and other regulatory authorities to market across the world.
CarboFix sales and marketing vice president Ron Szekely said: “This is another major breakthrough for CarboFix in its endeavor to bring the next revolution, using carbon fibers as the material of choice in orthopedic implants. We will continue to broaden our portfolio for the spine as we did with our full range trauma line.”
CarboFix is specialized in producing implants by using continuous carbon fibers reinforced polymer, as well as expandable implant technology.
The company’s product portfolio includes Piccolo Composite plating system, Piccolo Composite intramedullary nailing system and CarboClear pedicle screw system.
The firm produces Piccolo distal fibula plate, which is made by using continuous carbon Fibers reinforced polymer.
Fibula plates are available in two types, including anatomical distal fibula plate and one third tubular plate. These plates are FDA approved and CE marked.
CarboFix’s other products include Distal femur plate, Distal radius plate, Proximal humerus plate, one third tubular plate, diaphyseal plate and MTP fusion plate.




