UK-based medtech solutions provider Brainomix has launched the full suite of US Food and Drug Administration (FDA) cleared modules in its Brainomix 360 platform for stroke care in the US.
Brainomix 360 is a stroke artificial intelligence (AI) platform designed for both specialist and non-specialist stroke centres.
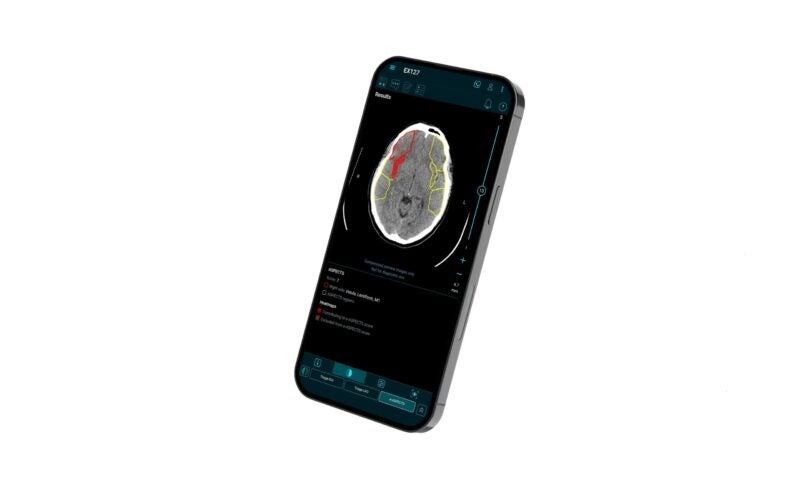
It is intended to aid physicians in making imaging-based therapy decisions at every stage of the stroke process, from basic imaging to more advanced imaging.
The stroke AI platform is powered by AI algorithms that read brain scans in real-time to support treatment and transfer decisions.
The US launch follows a series of FDA clearances for different modules including the e-ASPECTS module, which is intended for analysing non-contrast CT scans and automatically generate an ASPECTS score.
According to Brainomix, the Brainomix 360 platform aims to help more stroke patients receive the correct therapy at the right time and location.
Brainomix co-founder and CEO Michalis Papadakis said: “We are delighted to have the opportunity to introduce our Brainomix 360 platform to more and more US stroke networks, and to showcase the extensive validation of our technology, a good portion of which was conducted in the US at such institutions as the Mayo Clinic, Emory University, Mount Sinai in New York, and UCLA.”
Brainomix 360 e-CTP and Brainomix 360 e-MRI, two software modules that can support treatment decisions for thrombolysis and thrombectomy, were recently cleared by the FDA.
These clearances are important for late-window patients who arrive at the hospital more than six–12 hours after the stroke begins, the medtech solutions company said.
The stroke AI platform now includes two new notification technologies, Brainomix 360 Triage LVO and Brainomix 360 Triage ICH. These technologies are designed to warn physicians in real-time when there is suspicion of a bleed or large vascular occlusion (LVO).
Brainomix has plans to continue its expansion in the US with the rollout of its products across hospital networks in the country.
The company’s AI stroke software has undergone evaluation and validation in over 60 publications and has been implemented in more than 30 countries.


