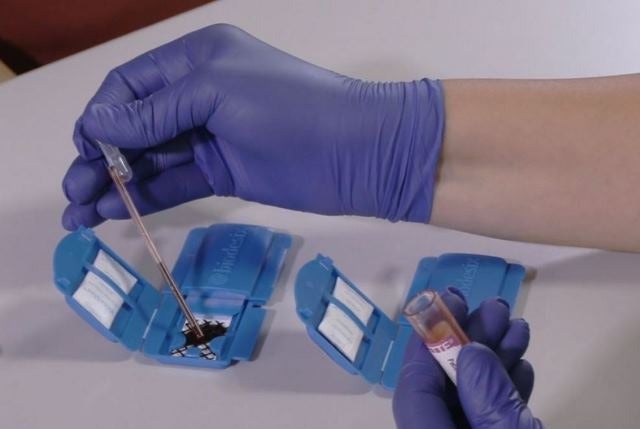
Biodesix announced it has been issued U.S. Patent Number 10,422,729, which covers the Biodesix Collection Device (BCD).
The new device offers improved ease of use in blood sample collection for diagnostic testing, compared to current standard methods.
“This device is intuitive and very easy to use,” remarked Susan Garwood, M.D., Pulmonary and Advanced Bronchoscopy Lung Cancer Specialist with Centennial Medical Center in Nashville, part of the HCA TriStar division. “The Biodesix Collection Device enables the opportunity for a fast, simple collection process. Enhancing patient comfort while retaining accuracy is of top concern for all professionals drawing patient blood samples.”
The BCD, currently in use with the Biodesix Nodify XL2™ test, replaces the need for processing samples used for proteomic testing on-site and shipping blood tubes, which often requires cold-chain logistics during transit to the testing laboratory. This reduces the need for processing equipment such as centrifuges in the clinic, improves sample stability during transit and eliminates the need for single-use, bulky shipping containers containing materials such as Styrofoam that are not only costly but also have a negative environmental impact.
“The BCD is designed for exceptional laboratory test performance and surpasses similar collection methods by combining multiple sample processing steps, including specimen collection and reproducible sample separation with ambient shipping. Specimen stability has been demonstrated with analytic measures of proteins using highly sensitive mass spectrometry methods,” said Gary Pestano, Ph.D., chief development officer at Biodesix.
“Our commitment to innovation continues to improve the customer experience,” commented Scott Hutton, chief operating officer at Biodesix. “We are as focused as ever on supporting healthcare professionals and their patients by providing increasingly valuable information to physicians using a noninvasive blood collection. The new BCD significantly reduces cost and environmental impact over current sample collection practices, and we are excited to not only use this for our own tests but to make it available for broad use across the industry.”
Biodesix is planning to use the new BCD in all current and future proteomic testing and also make the device available for purchase by labs and diagnostic companies wishing to use this novel technology for their own sample processing and testing. To see how the BCD is used to collect specimens for the Nodify XL2 test visit this link.
Source: Company Press Release






