Becton, Dickinson and Co. (BD) has received Health Canada authorisation for its rapid, point-of-care (POC), SARS-CoV-2 antigen test used on the BD Veritor Plus System, under an interim order.
In addition to the regulatory approval, the Canadian government has placed an order with the medical technology firm, for 7.6 million tests supplied through March 2021, to support the country’s Covid-19 testing strategy.
Canada Public Services and Procurement Minister Anita Anand said: “The Government of Canada continues to work diligently to provide Canadians with access to effective and efficient COVID-19 testing solutions.
“This new agreement with BD is critical to assisting the provinces and territories in their respective efforts to reduce the spread of the virus. We will continue to support Canadians through the COVID-19 pandemic.”
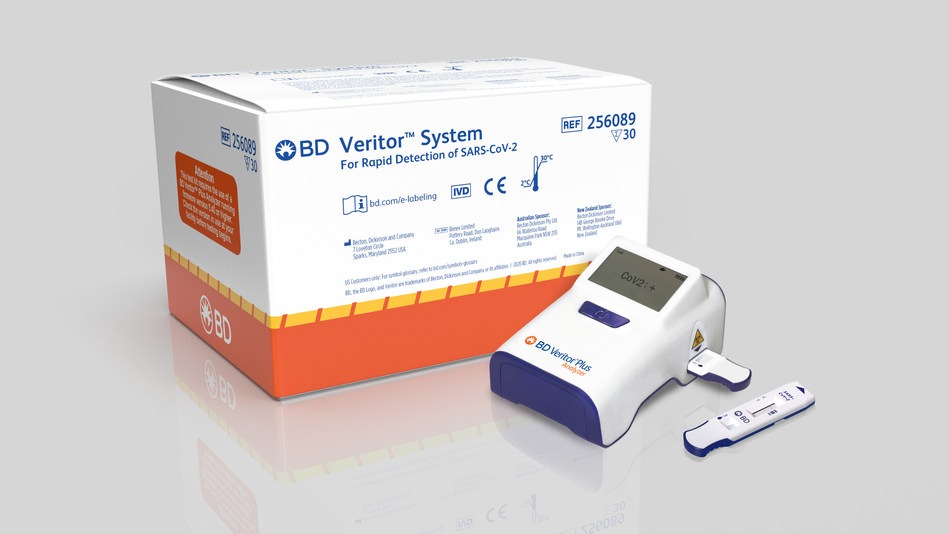
BD’s new rapid POC Covid-19 antigen test runs on BD Veritor Plus System
BD said that its new rapid antigen test has been designed to deliver results within 15 minutes, using the BD Veritor Plus System.
The system is already being used to detect Influenza A+B, Respiratory Syncytial Virus (RSV), and Group A Strep in Canada.
According to the company, the system offers customer traceability and reporting capabilities through the optional BD Synapsys informatics solution.
The new portable and rapid POC Covid-19 antigen test provides real-time results with improved turnaround time for Covid-19 diagnosis, and facilitates decision-making while the patient is still onsite.
Also, the test is expected to reduce the burden on testing laboratories, and requires a mid-nasal swab for sample collection, which offers less invasive option for patients compared to nasopharyngeal sample collection, said the company.
The test has been granted the US Food and Drug Administration (FDA) emergency use authorisation (EUA) and CE mark approval.
BD-Canada president Greg Miziolek said: “As the demands for SARS-CoV-2 testing are increasing in Canada, we are excited to bring another testing solution to the Canadian market.
“The ability to perform SARS-CoV-2 testing at the point-of-care and deliver results while the patient waits will be truly impactful to help relieve some of the pressures on the testing labs and quickly identify affected patients.
“We applaud the federal government for their proactive approach in planning for future needs with a centralized order for antigen detection tests. We look forward to partnering with Canada’s provincial health authorities to optimize the deployment of the BD Veritor Plus System to meet regional and local public health needs.”



