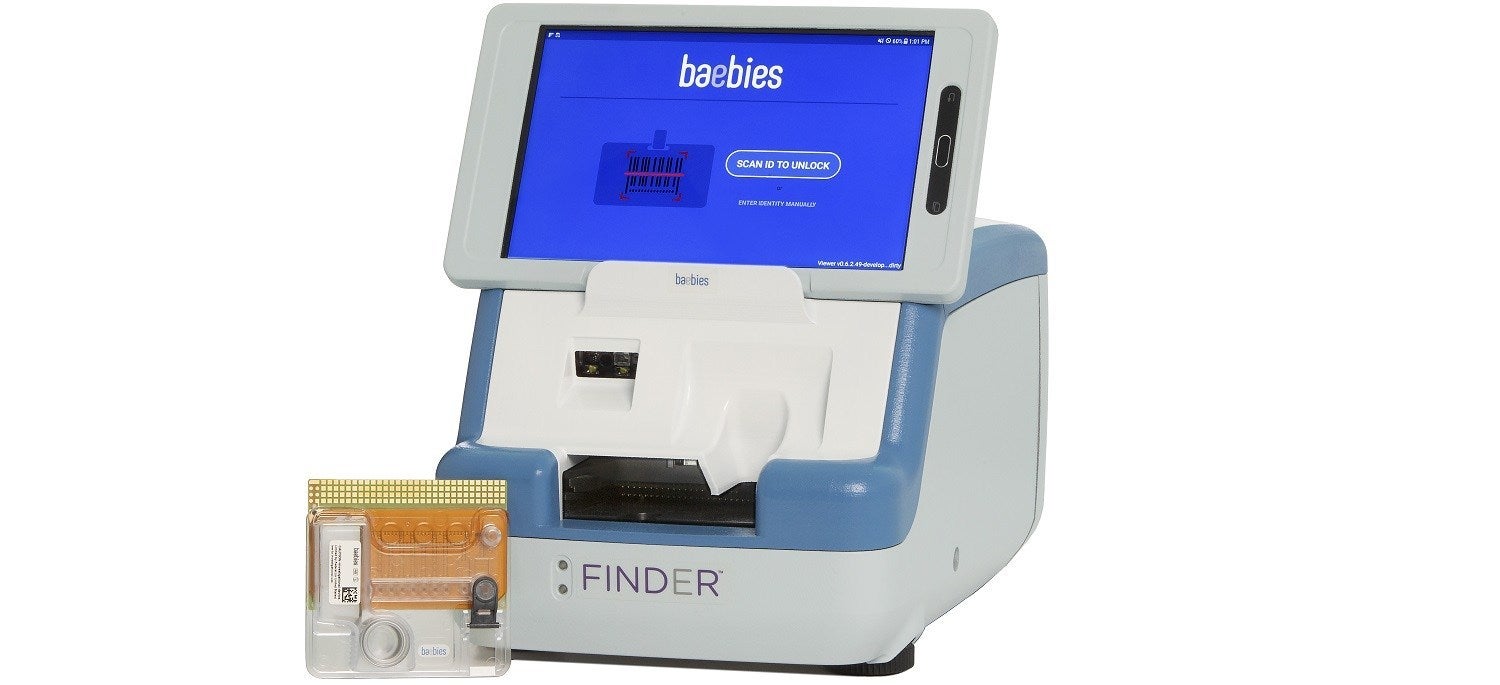
Baebies has obtained the US Food and Drug Administration (FDA) 510(k) approval for its rapid, point-of-care test to detect glucose-6-phosphate dehydrogenase (G6PD) deficiency.
The test is designed to work on the medical device company’s FINDER platform and comes with a compact instrument and a disposable cartridge.
It uses only one drop (50µL) of blood sample and delivers results within 15 minutes.
The FINDER platform test displays the G6PD enzyme activity results in units per gram of haemoglobin and adjusted male median values.
In December 2019, Baebies secured the CE mark approval for the FINDER platform, which leverages the company’s digital microfluidics (DMF) technology.
DMF technology involves a method of manipulating small droplets of liquid by electrical control of surface tension on a disposable cartridge.
It eliminates the need for mechanical pumps or valves for liquid handling, reduces the sample volume required, and provides rapid diagnostic results, said the company.
Baebies co-founder and CEO Richard West said: “This is a significant milestone not only for our company but for patients whose lives can be improved by detection of G6PD deficiency.
“FINDER performs rapid testing from just one drop of blood, making testing easier and more accessible. With G6PD as the first FDA-cleared test on our FINDER platform, we look forward to adding many additional types of tests to the versatile and multifunctional platform.”
According to the firm, deficiency in the G6PD enzyme is the world’s most common enzyme deficiency and causes premature destruction of red blood cells.
The most common clinical symptom related to G6PD deficiency is haemolytic anaemia, which occurs when red blood cells are destroyed faster than the body can replace them.
In addition to haemolytic anaemia, G6PD deficiency also causes mild to severe jaundice in newborns and can lead to kernicterus in some cases.
Baebies said that the early diagnosis of G6PD deficiency plays important role in enabling paediatric and adult patients to receive clinical care to manage symptoms.
Its G6PD deficiency test is the first among several other assays that are currently under development, for use on the FINDER platform.
The other assays are being developed to address unmet needs in haematology and infectious disease through multifunctional syndromic testing.
A team of researchers at Stanford University School of Medicine, led by professor Vinod K Bhutani, first evaluated the FINDER G6PD assay compared to known reference methods.
Bhutani said: “Recent updates to the AAP clinical practice guidelines on hyperbilirubinemia delineate an urgent need to identify G6PD deficient newborns so as to institute timely interventions such that educational, preventive and treatment options are offered soon after birth.
“Implementation of this mode of assay in nurseries will allow clinicians to know G6PD enzyme activity before a newborn is discharged from the hospital. In fact, FDA clearance of Baebies’ digital microfluidics assay introduces a new and important diagnostic solution to identify infants with G6PD deficiency.”




