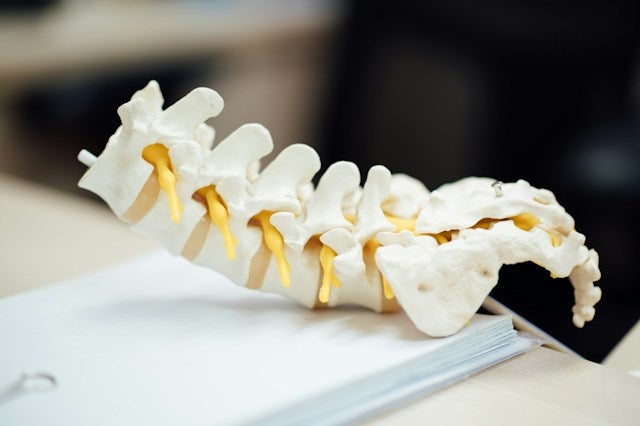
Aurora Spine, a provider of medical devices to improve spinal surgery, has secured the second US for its DEXA Technology Patient-Matched Implant Technology.
The company designed DEXA Technology to match a patient’s bone density and promote bone in-growth while maintaining the biomechanical structure and bone support.
The newly issued patent will be used to create implants that match the patient’s specific bone density based on a DEXA Scan/T-score, allowing for the best bone fusion treatment outcomes.
Aurora Spine said its DEXA Technology was created and patented to combine the essential design benefits of conventional machining and additive implant manufacturing.
Aurora Spine president and CEO Trent Northcutt said: “Aurora Spine’s first in the world and first to market DEXA Technology-based implant provides surgeons with the choice in selecting the matching density implant for their patient.
“Aurora’s second patent for bone quality-matching implants strengthens the company’s intellectual property portfolio significantly.
“Our proprietary DEXA Technology represents our continued commitment to delivering best-in-class implants that enable our surgeon partners to overcome common osteoporosis challenges faced in traditional procedures.”
In 2022, Aurora Spine launched its first DEXA Technology-based product, the DEXA-C interbody spacer line for anterior cervical fusion.
The DEXA-C interbody spacer is now well accepted by physicians as one of the most significant implant products to fight decreasing bone density due to ageing, said the company.
The same year, the Spinal surgery devices company received the US Food and Drug Administration (FDA) 510(k) approval for its DEXA SOLO-L spinal fusion system.
DEXA SOLO-L is a 3D printed, standalone anterior lumbar interbody fusion and lateral lumbar interbody fusion (ALIF & LLIF) device, developed based on its patented DEXA technology.
Aurora Spine chief technology officer Laszlo Garamszegi said: “Traditionally, medical devices for spinal procedures are designed as ‘one size fits all’ and assumes that every patient’s bone density is the same.
“The proprietary engineering behind our DEXA Technology is advancing the science of implant technology and ultimately helping improve patient fusion rates and overall clinical outcomes.
“These patents will allow us to create DEXA implants for use anywhere in the human body where bone fixation is needed providing patients with previously non-existing new treatment possibilities.”






