The EOGas 4 system is said to be suitable to sterilise reusable medical devices that are sensitive to moisture, heat, corrosion or radiation
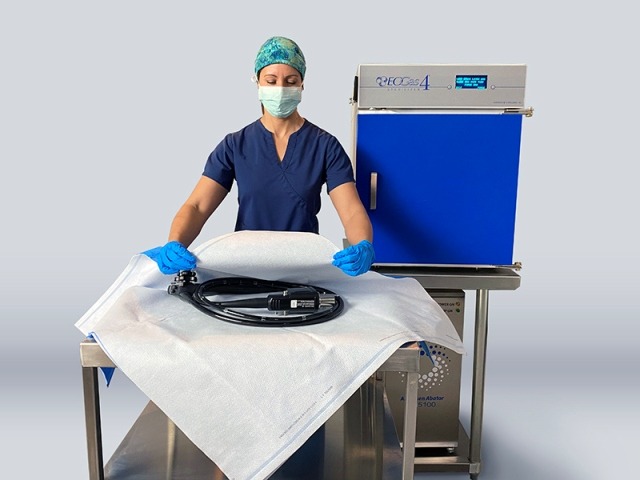
The FDA has approves Andersen Sterilizers’ EOGas 4 steriliser for duodenoscopes and colonoscopies. (Credit: Andersen Sterilizers)
Andersen Sterilizers has secured 510(k) clearance from the US Food and Drug Administration (FDA) for its EOGas 4 ethylene oxide (EO) gas steriliser and associated accessories for terminal sterilisation of duodenoscopes and colonoscopies.
The EOGas 4 system has been labelled for the sterilisation of reusable medical devices, which are sensitive to moisture, heat, corrosion or radiation.
In 2015, the EOGas 4 was first approved for surface sterilisation of a wide range of instruments, as well as for sterilisation of endoscopes with < 1100 mm in working length.
The EOGas 4 system is used to sterilise different endoscopes
The EOGas 4 system is designed to be used for sterilisation of different endoscopes such as bronchoscopes, bronchovideoscopes, cystoscopes, ureteroscopes, choledocoscopes, and gastroscope.
The FDA’s new approval allows to use EOGas 4 system for sterilisation of endoscopes, including duodenoscopes and colonoscopies, with > 1100 mm working length and maximum lumen length of 3530mm and minimum lumen diameter of 1.2mm.
The EOGas 4 process is said to sterilise duodenoscopes and colonoscopes in wrapping materials suitable for EO sterilisation, thereby offering six-months of sterile shelf life for endoscopes after sterilisation.
Wrapping materials such as Tyvek / plastic pouches or Sterisheet sterilisation wraps are suitable for sterilisation.
Andersen Sterilizers president Dr William Andersen said: “Prior to clearance of the EOGas 4 system in 2015, we had already started working closely with FDA on the requirements for duodenoscope and colonoscope sterilisation.
“This clearance reflects the FDA’s latest validation expectations for terminal sterilization of lumened devices, including those with elevator mechanisms. We also received clearances on chemical indicators, biological indicators, and packaging that maintains sterility of endoscopes for up to 6 months after processing.”
