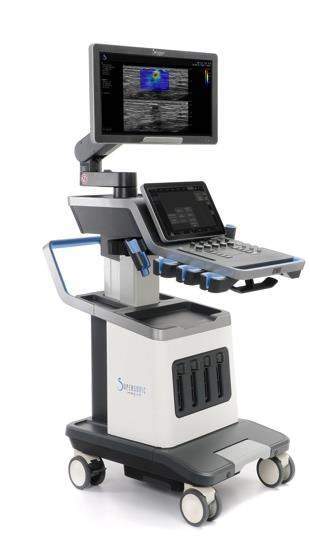
The new ultrasound system features latest UltraFast imaging technology, which will enhance full range of imaging modes developed by SuperSonic Imagine for better diagnostic performance.
SuperSonic’s ultrasound system is also provided with SonicPad touchpad that will simplify the user experience.
SuperSonic Imagine CEO Michèle Lesieur said: “These approvals not only confirm that the Aixplorer MACH 30 is recognised for its quality, performance, and compliance with American and European regulatory standards, but it also clears the way for marketing of the system in the US, European member states, and many other countries around the world.
“We are confident this new innovative platform will offer clinical value which supports our growth ambitions in the Global Ultrasound Market.”
Aixplorer system also offers Angio PL.U.S imaging mode for imaging microvascularization of lesions, as well as triplex mode that aggregates three diagnostic data sets in a single examination.
The Aixplorer MACH 30 ultrasound system is said to apply an advanced ShearWave elastography (SWE) solution, dubbed ShearWave PLUS.
The ultrasound system provides users with real-time, quantitative, and reproducible 2D and 3D visualization. It also evaluates tissue stiffness.
Aixplorer system can accurately distinguish benign and malign lesions, enabling to reduce multiple false positives and unnecessary biopsies.
SuperSonic Imagine sales chief business officer Kurt Kelln said: “During clinical evaluations performed in the US, we received enthusiastic customer feedback from those who used the Aixplorer MACH 30 and its touchpad.”
Based in Aix-en-Provence of France, SuperSonic Imagine is engaged in the designing, development and commercialization of advanced ultrasound systems under Aixplorer brand.
The firm also provides ultrasound technologies such as Angio PLanewave UltraSensitive (PL.U.S) imaging and Needle PL.U.S. SWE, which enable physicians to visualise and analyse the stiffness of tissue in real time.
The UltraFast Doppler aggregates colour flow imaging and pulsed wave Doppler into one simple test, offering physicians with the results of both simultaneously.






