Peregrine is suitable for use alongside the traditional 0° rigid endoscope or as the main endoscope in the operating room
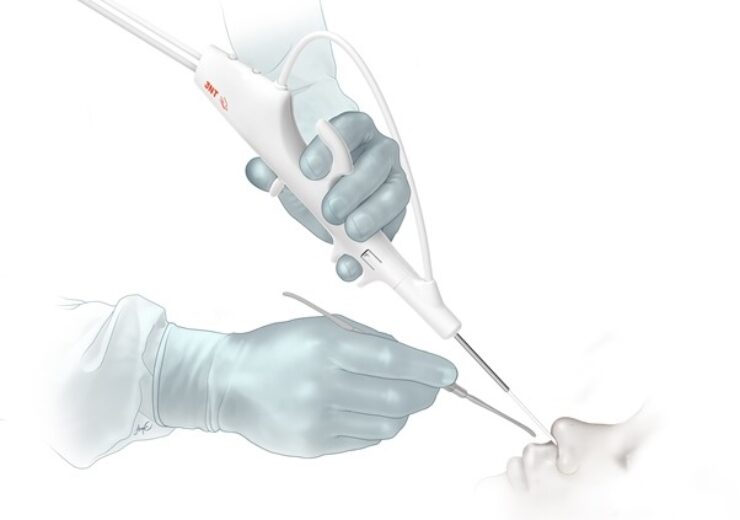
The FDA has approved 3NT Medical’s single-use Peregrine sinus endoscope. (Credit: Business Wire)
3NT Medical has secured approval from the US Food and Drug Administration (FDA) for its Peregrine Drivable ENT Scope.
The new single use sinus endoscope will enable ENT surgeons to access and view deep intra-sinus anatomy with minimal resection.
The device allows to visualise anatomic landmarks up-close with enhanced image quality and minimal resection, both intra and post-operatively.
Its novel mechanics helps the surgeon to angulate the device’s 2.3mm tip sharply, as well as then advance it further in the direction of the turn and all through single-hand operation.
Peregrine is suitable for use alongside the traditional 0° rigid endoscope, or as the main endoscope in the operating room.
The small-footprint video console to which Peregrine is linked negates the need for bulky and expensive endoscopy towers that traditional endoscopes require, said the company.
According to the company, Peregrine was able to view 100% of the frontal and sphenoid sinuses and 97.1% of the maxillary sinus compared to 45.3%, 53.3% and 39.4% for 4mm rigid endoscopes ranging from 0° to 70°.
3NT Medical CEO Ehud Bendory said: “Assessing the sinuses from a distance is now a thing of the past with Peregrine. This new scope joins our commercially-available Colibri Micro ENT Scope for otologists, and demonstrates that 3NT Medical is executing on its vision of creating a portfolio of endoscopes designed specifically for ENT use.
“We designed Peregrine exclusively for ENT surgeons to enable a better surgical experience for them as they strive to provide better outcomes and an enhanced experience for their patients.”
3NT Medical is engaged in the development of endoscopic solutions for otorhinolaryngology applications across the world.
