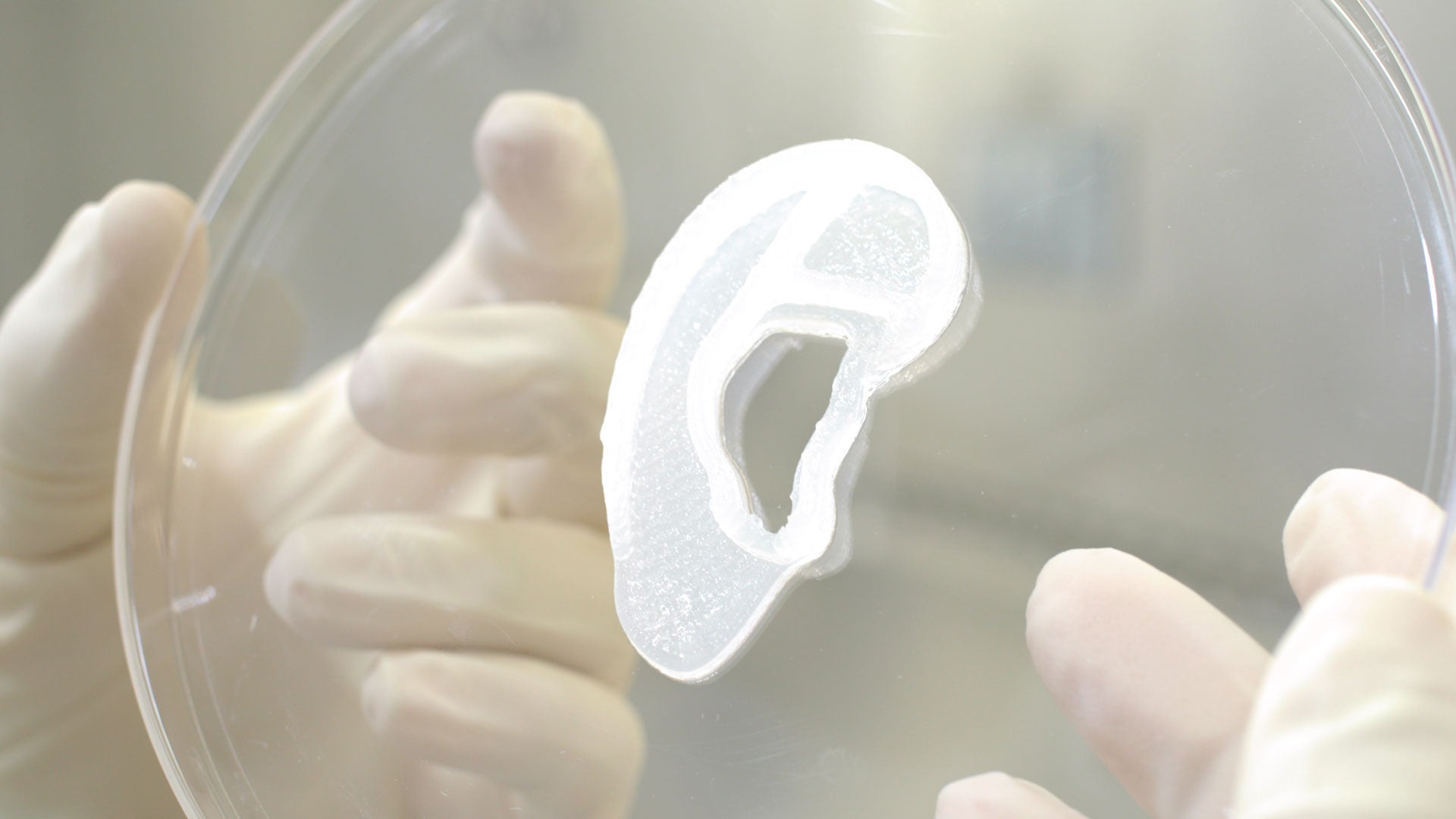
Regenerative medicine company 3DBio Therapeutics and the Microtia-Congenital Ear Deformity Institute have conducted a human ear reconstruction using the AuriNovo implant.
AuriNovo is a 3D-bioprinted implant that was developed by 3DBio, utilising a patented technology that used the patient’s own tissue to construct an implantable ear.
The procedure was performed at Microtia-Congenital Ear Deformity Institute in San Antonio, Texas by Arturo Bonilla.
The procedure in the first-in-human Phase 1/2a clinical trial is examining the safety and preliminary efficacy of AuriNovo for patients with microtia, a rare congenital abnormality.
AuriNovo is a patient-specific living tissue implant for surgical restoration of the outer ear in persons born with microtia Grades II-IV.
The construct is printed according to the 3D scan of the size and shape of the patient’s opposite ear for implantation.
AuriNovo is designed to provide an alternative treatment to rib cartilage grafts and synthetic materials conventionally used to reconstruct the outer ear of microtia patients.
The firm said the device received Orphan Drug and Rare Pediatric Disease Designations from the US Food and Drug Administration (FDA).
3DBio Therapeutics chief executive officer and co-founder Daniel Cohen said: “This is a truly historic moment for patients with microtia, and more broadly, for the regenerative medicine field as we are beginning to demonstrate the real-world application of next-generation tissue engineering technology.
“It is the culmination of more than seven years of our company’s focused efforts to develop a uniquely differentiated technology platform meeting the FDA’s requirements for therapeutic manufacturing of reconstructive implants.
“We believe that the microtia clinical trial can provide us not only with robust evidence about the value of this innovative product and the positive impact it can have for microtia patients, but also demonstrate the potential for the technology to provide living tissue implants in other therapeutic areas in the future.”



