All articles by Vidya Sagar
Philips expands network of clinical partners to set new standard of care for the early diagnosis and treatment of lung cancer
Royal Philips (NYSE: PHG, AEX: PHIA), a global leader in health technology, today provided an update on the growing global clinical…
STAAR Surgical Announces U.S. FDA Approval of EVO Visian® Implantable Collamer® Lenses
STAAR Surgical Company (NASDAQ: STAA), a leading developer, manufacturer and marketer of implantable lenses and companion delivery systems for the…
Sky gets FDA nod for new variant of geko device for venous insufficiency
Sky Medical Technology has received the US Food and Drug Administration (FDA) 510(k) approval for its new-generation geko device to…
FDA Clears Cutera’s AviClear™ Acne Device
CUTERA, INC. (Nasdaq: CUTR) (“Cutera” or the “Company”), a leading provider of dermatology solutions, today announced the U.S. Food and…
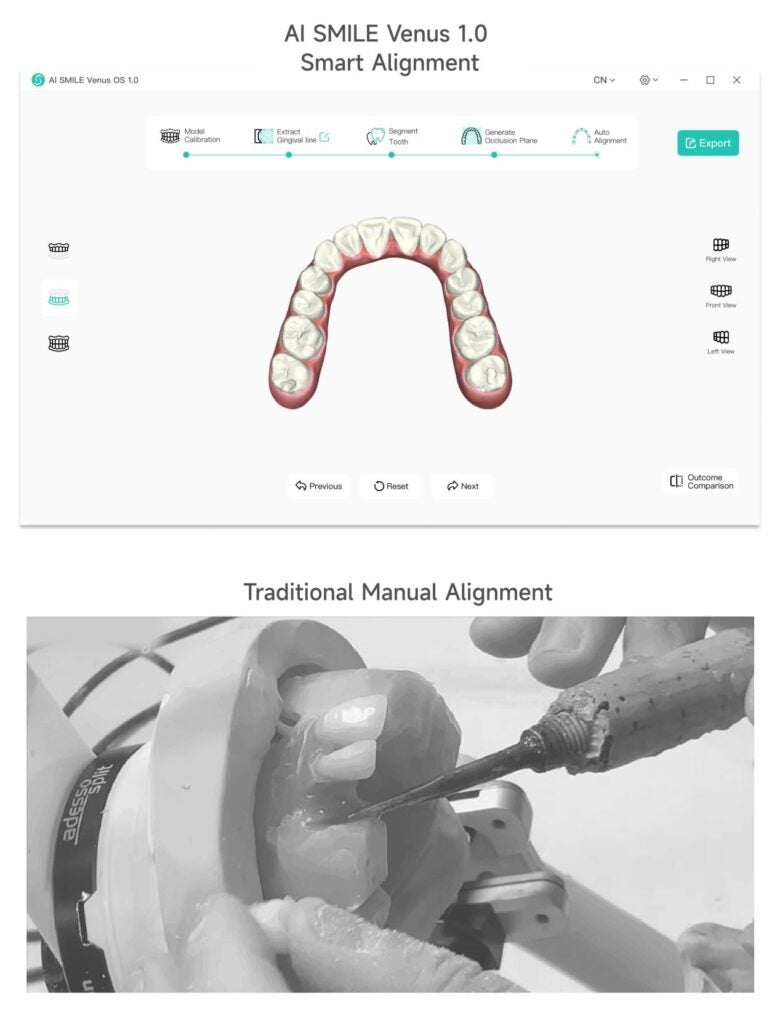
AI Firm WEIYUN AI & Robotics Group Launches Orthodontic Smart Alignment System Venus 1.0
Recently, the leading surgical robotic and AI customerized industrial Internet company – Weiyun AI&Robotics Group has successfully developed AI SMILE…
Medtronic begins patient implants for tibial neuromodulation device study
Medtronic has completed the implantation of first patient with its experimental tibial neuromodulation (TNM) device to treat urinary incontinence. The…
Sight Sciences Announces First Patient Treated in TRIDENT European Trial to Evaluate the OMNI® Surgical System in Pseudophakic Eyes with Open-Angle Glaucoma
Sight Sciences, Inc. (Nasdaq: SGHT), an eyecare technology company focused on creating innovative solutions intended to transform care and improve…
New Study Finds Surface Imaging Provides Highly Accurate Models of The Body for Scoliosis Surgeons
A new study that includes researchers at Hospital for Special Surgery (HSS) in New York City has found that a technique called…
Cerus Endovascular gets FDA 510(k) approval for 027 micro-catheters
US-based neuroradiology devices maker Cerus Endovascular has received the US Food and Drug Administration (FDA) 510(k) approval for its 027…
TendoNova wins FDA Clearance for Its New Microinvasive Ocelot Surgical Tool
TendoNova, an emerging leader in microinvasive sports medicine procedures, is pleased to announce the FDA 510(k) clearance of its new…