The RefleXion X1 machine combines high-quality CT imaging with a linear accelerator for improved tumour localisation
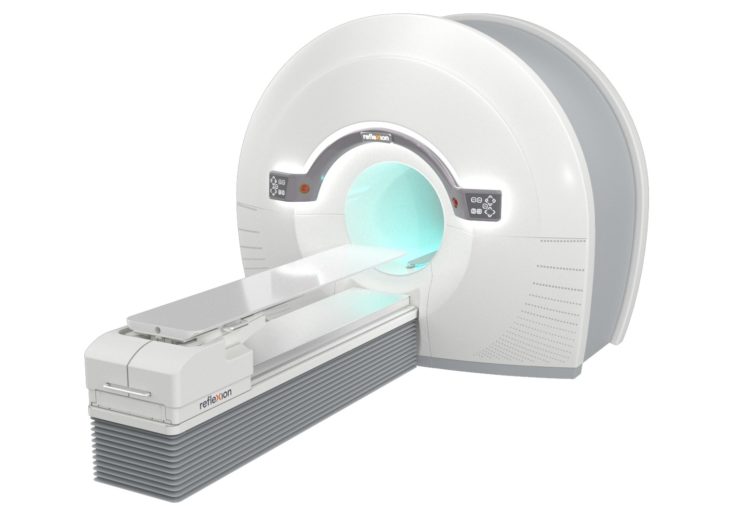
The RefleXion X1 is the only machine that combines high-quality CT imaging with a linear accelerator for better tumour localisation. (Credit: Business Wire)
US-based medical equipment manufacturer RefleXion Medical has secured approval from the US Food and Drug Administration (FDA) to market its RefleXion X1 machine.
The FDA cleared the Reflexion X1 for the delivery of stereotactic body radiotherapy (SBRT), stereotactic radiosurgery (SRS) and intensity modulated radiotherapy (IMRT).
The company claims that the X1 is the only machine to combine high-quality, fan-beam computed tomography (CT) with a linear accelerator to diminish motion noise that can affect the accurate dosing of a patient’s tumour.
X1 machine rotates up to 60 times faster than other linear accelerators
The system’s innovative design revolves up to 60 times quicker than existing radiotherapy machines and modulates the firing of radiation doses from 100 points per beam station.
RefleXion stated that these combined improvements may reduce some of the side effects related with radiotherapy by letting radiation oncologists to better localise the tumour, decrease patient setup errors and accurately provide dose to complex tumour targets while evading neighboring normal structures.
RefleXion CEO and president Todd Powell said: “We are at the forefront of an enormous change in expanding the use of radiotherapy from a treatment solely for early-stage cancer patients to an entirely new group of patients who need it most, those with advanced stage cancer.
“This initial marketing clearance of our RefleXion X1 machine is a steppingstone on the path to our goal of providing BgRT as a novel treatment modality that will expand the overall radiotherapy market significantly.
Based in California, RefleXion is also involved in the development of a biology-guided radiotherapy system (BgRT), that aims to bring multi-tumour precision radiotherapy to treat all stages of cancer.
According to RefleXion, the X1 machine with BgRT will address the technical limitations that presently restrict radiotherapy to one or two tumours.
The RefleXion X1 BgRT capability requires 510(k) clearance and is not available for sale.
In April 2018, RefleXion Medical had raised over $100m series C financing, which would be used to commercialise its BgRT for cancer treatment.
