Michigan-based medical technologies firm Stryker announced that US Food and Drug Administration (FDA) has granted Premarket Approval (PMA) for its Neuroform Atlas Stent System.
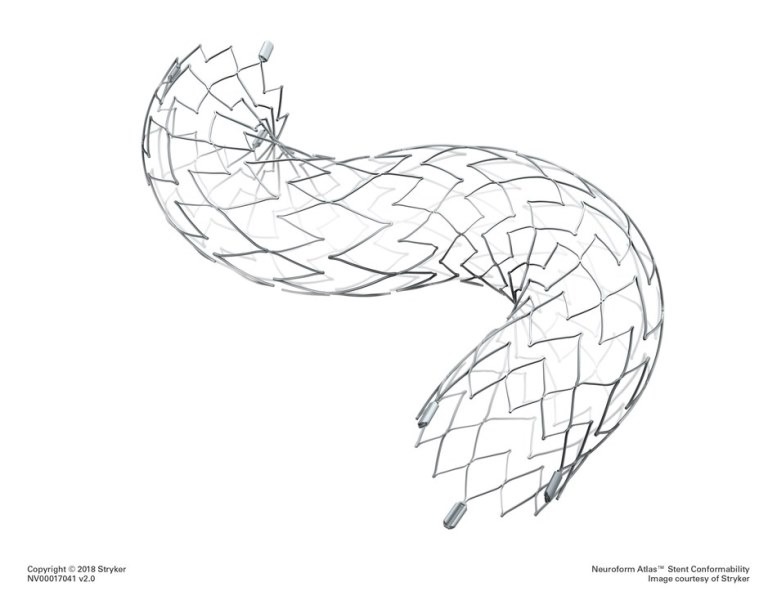
Image: Neuroform Atlas Stent System is a self-expanding nitinol stent used in combination with metal coils. Photo: Courtesy of PRNewsfoto/Stryker.
Strykar said that the Neuroform Atlas Stent System forms the second aneurysm adjunctive stent to be granted PMA approval for the treatment of wide-neck, intracranial aneurysms in combination with embolic detachable coils.
The stent system was previously approved for restricted use in specific hospitals with institutional review board approval, under a humanitarian device exemption. Based on clinical trial evidence proving the efficacy of the device, the system was granted PMA approval.
University of Pittsburgh Medical Center NeuroEndovascular Fellowship program director Dr. Brian Jankowitz said: “Enhanced stent conformability, a low-profile delivery system and high deployment accuracy even in distal anatomy puts Neuroform Atlas in a category of its own.
“This product is changing my clinical practice by allowing more patients with difficult aneurysms an option at endovascular treatment while improving the quality and safety of treatment.”
Stryker said that its Neuroform Atlas Stent System is a self-expanding nitinol stent used in combination with metal coils to pack weakened blood vessel sacs called aneurysms within the brain. The stent is placed across the aneurysm neck to hold metal coils and occlude the aneurysm.
The Neuroform Atlas Stent System is directed for use with neurovascular embolization coils in the anterior circulation of the neurovasculature for the endovascular treatment of patients.
The stent is indicated for use in patients aged 18 years or above with saccular wide-necked (with neck ≥ 4mm or a dome-to-neck ratio of < 2) intracranial aneurysms arising from a parent vessel with a diameter of ≥ 2.0 mm and ≤ 4.5 mm.
Stryker Neurovascular division president Mark Paul said: “Proving the safety and efficacy of our products through landmark clinical trials is a top priority and key differentiator for Stryker. Meaningful clinical data enables our market leading products to better serve patients suffering from debilitating cerebrovascular disease.
“PMA approval of Neuroform Atlas Stent System is a significant milestone in providing world class technology to our physicians.”
