Orthofix Medical Inc. is a global medical device with a spine and orthopedics focus
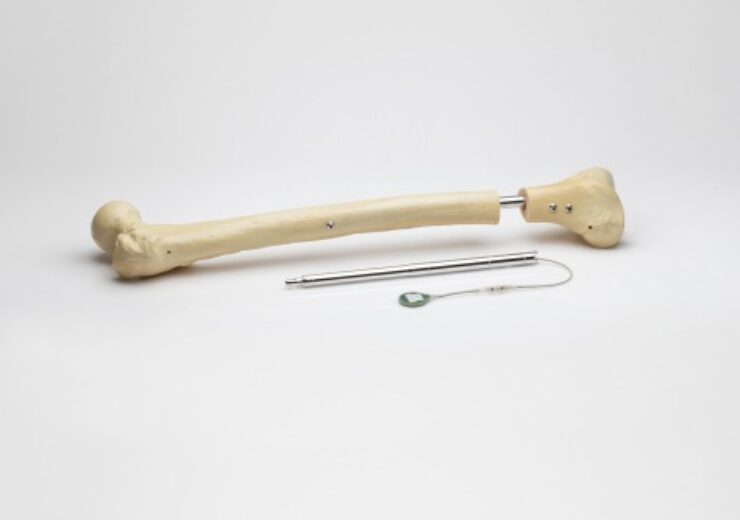
Image of the Fitbone intramedullary lengthening system for limb lengthening of the femur and tibia bones. (Credit: Business Wire.)
Orthofix Medical Inc. (NASDAQ:OFIX), a global medical device company with a spine and orthopedics focus, today announced the first U.S. pediatric patient implanted with the Fitbone™ intramedullary lengthening system implant for limb lengthening of the femur and tibia bones.
The Fitbone system is the only intramedullary limb lengthening solution cleared by the U.S. Food and Drug Administration (FDA) for pediatric and adult use and is available in a 9mm, 11mm and 13mm sizes to address the specific needs of patients. The system is now compatible with the OrthoNext™ digital platform, the only software tool in the market for deformity analysis and preoperative planning for pediatric and adult orthopedic procedures. The OrthoNext software features a reverse planning method module that simulates the target position, osteotomy level and blocking screw placement — enabling a more accurate preoperative assessment.
“Deformity correction and limb lengthening can be challenging to plan,” said Dr. David Frumberg, an orthopedic surgeon who directs the Limb Restoration and Lengthening Program in New Haven, Conn., and performed the first U.S. pediatric patient implant. “The OrthoNext digital planning module has really helped streamline my preoperative planning, specifically when using the Fitbone system in adolescent patients where the exact limb alignment is crucial.”
“At Orthofix, we are committed to being at the forefront of solutions like the Fitbone intramedullary lengthening system that improve outcomes and quality of life for patients needing limb lengthening,” said Orthofix President of Global Orthopedics Paul Gonsalves. “We are pleased to be able to offer a technology that enables surgeons to provide exceptional care for pediatric patients.”
The Fitbone system consists of the implanted intramedullary nail, a subcutaneously implanted receiver, and an external control set that enables the patient or their caregiver to manage the distraction phase at home. The system is designed to provide accurate and controlled limb lengthening, with more than 3,500 cases performed in 15 countries since its development. With appropriate preoperative planning, it allows achievement of axial and torsional bone alignment intraoperatively, as part of the limb-lengthening procedure.
The Fitbone intramedullary lengthening system is available through a U.S. Food and Drug Administration 510(k) clearance and in European Countries under the CE Mark approval.
Orthofix is the only orthopedic company that offers a comprehensive portfolio of both internal and external fixation solutions for limb reconstruction and deformity correction. For those attending the American Academy of Orthopaedic Surgeons annual meeting in San Diego, please visit booth 2517 to learn more about the Fitbone intramedullary lengthening system.
Source: Company Press Release
