Development of Revolutionary Next-Gen Electrotherapy Device Fully Complete
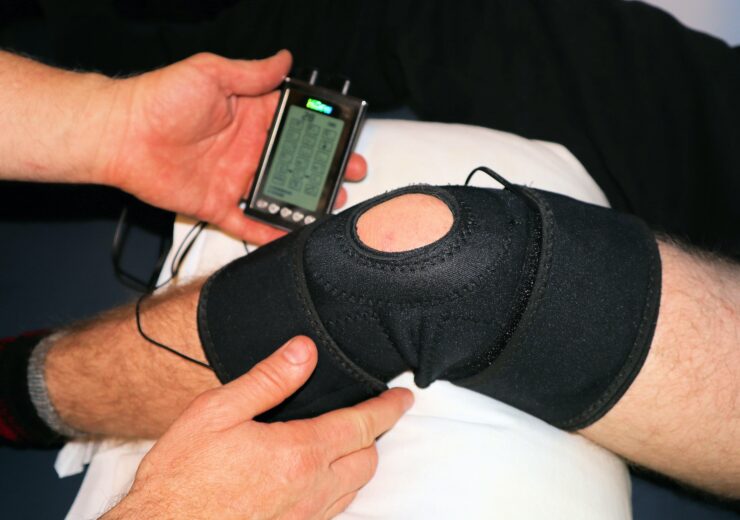
Company expects to start filling orders in Q1 2023. (Credit: Terry Shultz P.T. on Unsplash)
InvestorsHub NewsWire — Electromedical Technologies (“Electromedical” or the “Company”), a pioneer in the development and production of bioelectronic devices designed to relieve chronic, intractable, and acute pain through frequency and electro-modulation, is very excited to introduce its new next-generation flagship device, the WellnessPro Infinity.
“The WellnessPro Infinity will set an entirely new standard in the Bioelectronics marketplace” remarked Electromedical Founder and CEO, Matthew Wolfson. “There’s nothing like it on the market in terms of capability, flexibility, ease of use, and doctor-patient interface. And most important of all – Patient results. ”
The NEW WellnessPro Infinity device features:
- One compact device that encompasses ALL electrotherapy modalities.
- Can produce any frequency, any wave form (typical or atypical), any level of modulation
- Designed to support: TENS, Microcurrent, IF, EMS, PEMF, CES, VNS, PNS, COLD Laser, POD Synchronization, IDNA, Deep Pluse, Cloud access and much more.
- Unprecedented accuracy.
- Easiest to use electrotherapy device on the market.
- New Doctor-Patient Portal for updates and personalized treatments.
Wolfson added, ” I want to thank our shareholders for the continued support, everyone at EMED and especially our incredible engineering team in Europe, for the hard work, innovation and long hours our team put in, to create a device we believe will help millions of people live happier healthier Pain Free lives and help battle the Opioid epidemic. Now that we have completed development of our NEW flagship model by October as planned, the next step is electrical laboratory safety testing. After that, it will be production ready. Once production is underway, we will begin taking pre orders. Our goal is to start shipping units next quarter. POD development is going as planned and we look forward to sharing progress in our upcoming press releases soon. Our goal is to go to market with the WellnessPro Infinity first, then shortly after releasing the POD upon FDA 510k clearance.”
Source: Company Press Release
